A biomolecular approach to Neanderthal technology
Published in Ecology & Evolution

Maybe it was the usual quality of research presented at ESHE. Or maybe it was that this year was in Tübingen, home to one of the few research groups that has dedicated as much time to studying Neanderthal birch tar as I have. Either way, I felt a sense of determination and started writing.
Birch bark tar is the earliest known adhesive. Used as a glue on Neanderthal stone tools at least 200,000 years ago. It pre-dates anything similar from anatomically modern human contexts by a considerable margin. It also does not occur naturally (at least not in the quantities found archeologically). Unlike other ancient adhesives, such as pine resin, that oozes - sticky and viscous - straight from the tree, birch bark tar must be manufactured. And it must be made by manipulating fire in specific ways. How Neanderthals could have discovered birch tar, and then turned it into a perennial innovation has caused some debate in Palaeolithic archaeology.
There are multiple ways Neanderthals could have been making tar. While every method involves using fire to transform white papery birch bark into black sticky goo, some methods may be considered more or less 'complex' than others. A lot of that depends on how you measure complexity in ancient technological processes, but that is in part the story of another article (Kozowyk et al., 2023). So, if we want to understand more about the technological prowess of Neanderthals, it would help to know how they were making their tar.
Herein lies a problem. There are not a lot of physical remains of birch tar production from the Middle Palaeolithic. There are two flint flakes containing tar from Italy (Mazza et al., 2006), two small lumps of tar from Germany (Koller et al., 2001), and a single flint flake partly covered in tar from the Netherlands (Niekus et al., 2019) (Fig. 1). There is no clear evidence, nor physical traces, of the actual production processes.

To understand how tar was made, archaeologists must look at clues within the tar itself. Using Gas Chromatography-Mass Spectrometry (GC-MS), specific chemical compounds, or 'biomarkers' are usually used to identify the source of organic residues on stone tools. Fats and oils, resins and tars all have unique chemical fingerprints depending on their source. These biomarkers may also be altered by both natural and artificial forces. This provides the possibility of reconstructing long lost details such as heating temperature and duration, or the availability of oxygen during production. For example, tars made above ground were likely exposed to heat for less time, but with more oxygen than tars made below ground (Fig. 2). They should therefore have unique biomolecular compositions.
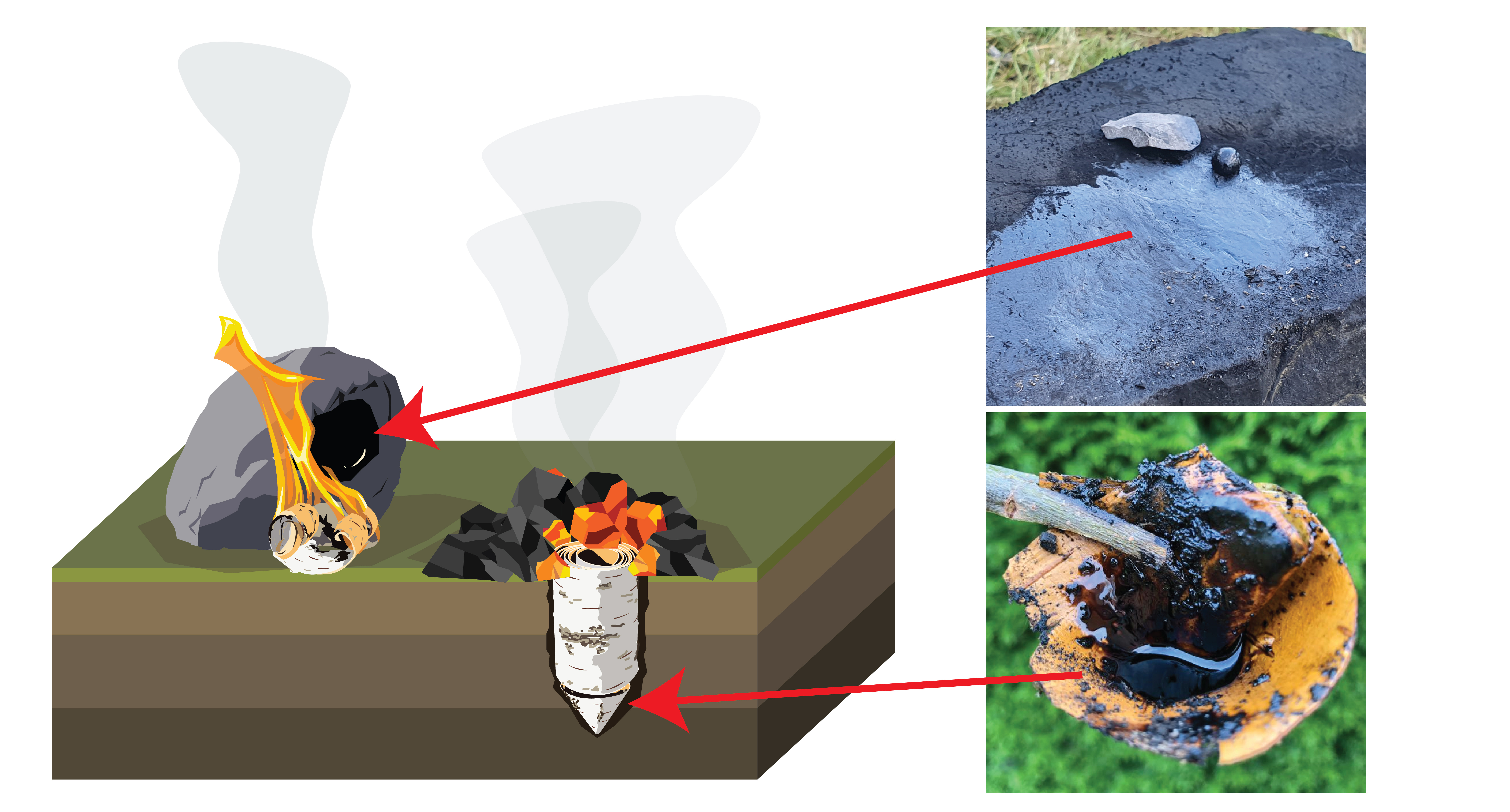
I frequently find myself referring back to a paper written by Maxime Rageot et al. (2019) on biomolecular approaches to reconstructing Neolithic tar. It contains an abundance of experimental and biomolecular information on prehistoric tar making. However, there are two problems. 1) Neanderthals did not use ceramic containers, and 2) trying to glean additional information and compare chromatograms from different publications is extremely difficult. There is no easy way to fit multiple chromatograms full of peak labels onto a single page and make them readable. Considerable amounts of mass spectra data are also often left out altogether. If you want to know what molecule is represented by that one peak and it doesn't have a label, or isn't mentioned in the caption, then you are out of luck.
That sparked the idea for this paper. Can Rageot et al.’s key biomarkers also be used to differentiate Palaeolithic tar production strategies? And subsequently, can people please share their GC-MS data? I started wracking my head around names like lupa-2,20(29)-dien-28-ol and lupa-2,20(29)-dien-28-oic acid, and sent an abundance of emails to my co-author, Liliana Baron about the presence or absence of these compounds in the experimental samples she had already put through the GC-MS. Unfortunately, we didn’t have time to run new samples, so we selected the best representation of relevant production methods from experiments that were already completed.
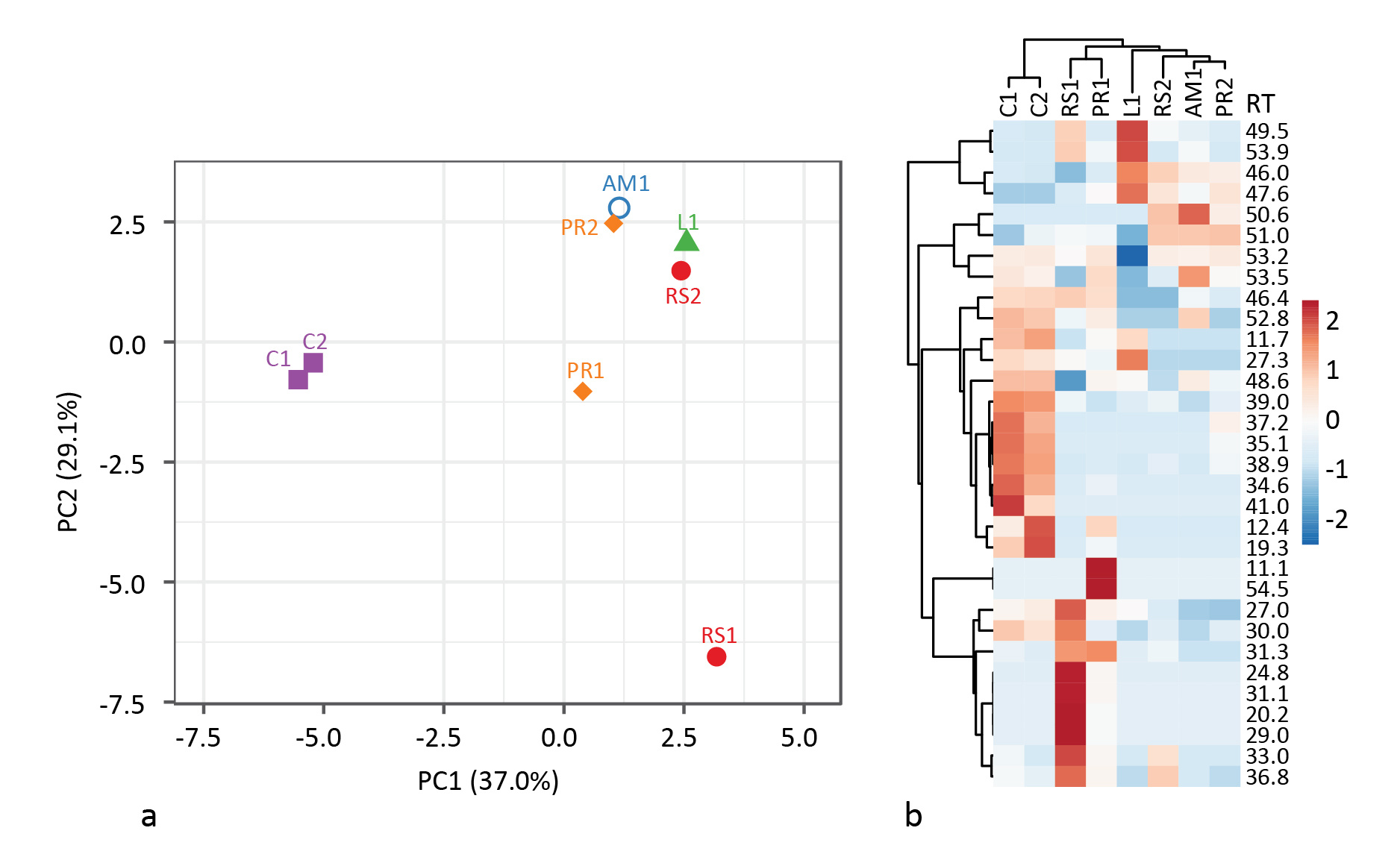
In the end, it was difficult to differentiate Palaeolithic tar production strategies based on the same biomarkers used by Rageot et al. for the Neolithic. This is likely in part due to the greater variation in heating conditions when not using ceramics. Strict oxygen and temperature control is impossible, and tar can be collected from different locations inside the bark roll, resulting in widely varying results even when using the same method. And partly due to the small sample size. Principal component analysis of the relative abundance of select biomarkers based on GC peak area % shows where differences between methods can be found (Fig. 3). Unfortunately, there is also appreciable intra-method variation. More samples should help in that regard.
But all is not lost. Schmidt et al. (2023) suggest using FTIR and have had interesting results re-analyzing the tar from Königsaue, Germany. And there are many more experiments that could be done to help determine key variables. Yet, there remains a problem with researching anything Palaeolithic and organic. The sample size is incredibly small. Individual institutions can create reasonable experimental reference collections by themselves. But they can never test everything. The influence of soil type, or tree growing conditions may cause small changes to the biomolecular composition of tar and be unique to each location. And nothing short of a miracle will dramatically increase the number of Palaeolithic tar artefacts. The need to share data, then, becomes even more apparent.
As I am preparing to embark on the train to Aarhus, exactly 12 months later, I am looking forward to what this year's ESHE conference will bring. I'll be checking my email occasionally from the back row.
Acknowledgements: This research was supported as part of the Ancient Adhesives project, funded by the European Research Council under the European Union’s Horizon 2020 research and innovation programme Grant Agreement No. 678 804151 (Grant holder Geeske Langejans). Experiments were conducted at Masamuda, the Netherlands.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in