
Stem cells (SCs) ensure tissue morphogenesis during development and their physiological turnover during adult life. SCs are also critical for the regeneration of adult tissues after injuries. Glandular epithelia, including the mammary and the prostate gland are composed of basal cells (BCs) and luminal cells (LCs). We previously developed new lineage tracing methods to study the development, homeostasis, repair, cancer initiation and growth of these tissues. Genetic lineage tracing has demonstrated that these two tissues initially arise from multipotent SCs that are replaced after birth by distinct types of lineage-restricted unipotent SCs. We recently also found that in pathological conditions such as oncogene expression and regenerative stimuli can re-induce adult basal SC (BaSC) multipotency in these lineage-restricted unipotent SCs. However, the molecular mechanisms regulating SC plasticity, promoting or restricting multipotency of glandular epithelial SC are poorly understood.
Gene Signatures associated with BaSC Multipotency
To unravel these mechanisms, we characterized the gene signatures of BaSCs under different conditions associated with multipotency: mammary gland progenitors during their initial stage of embryonic development1, transplantation of BaSC into the mammary fat pad2, BaSC following oncogenic PIK3CA expression3, BaSC following LC ablation4, and Prostate BaSC during postnatal development5.
Collagen and ECM Stiffness are key Regulators of SC multipotency
A comparative analysis of BaSC multipotent signatures across these different conditions associated with multipotency in BaSCs of both the mammary gland and prostate (development, transplantation, oncogenic mutation, LC ablation) identified a common gene signature composed of high level of different collagens (Col1a1, Col1a2, and Col3a1). To assess the role of collagen in the regulation of multipotency, we assessed the fate of BaSC by lineage tracing using MG and prostate organoids embedded in different concentration of collagen. Our data revealed that high concentration of collagen 1 promotes BaSC multipotency in prostate and mammary gland. To assess whether rising Col1 concentration promotes BaSC multipotency by increasing ECM stiffness, we embedded MG organoid and prostate organoids in polyethylene glycol gels (PEGs) presenting different stiffness. Our data show that higher stiffness promotes BaSC multipotency.
Mechanistic Insights
To understand the mechanisms by which collagen and stiffness promote multipotency, we performed single-cell RNA sequencing (scRNA-seq) to profile MG organoids embedded in different conditions promoting or not multipotency. Our data showed that high collagen concentration or high stiffness induces the differentiation of BaSC into LCs. Bioinformatics analysis together with in situ characterization by immunostaining indicate that the activation of BaSC multipotency induced by Collagen and ECM stiffness is a progressive process associated with the emergence of transient hybrid states that eventually differentiate into LCs after losing their BC identity.
Col1 binding to the integrin α2β1 activates a signaling cascade which involves the focal adhesion kinase (FAK) activation and the related downstream pathway to promote different cellular responses. β1-integrin blocking antibody and pharmacologically inhibition of β1 and FAK signaling inhibited Collagen and ECM stiffness induced BaSC multipotency. Interestingly, scRNA-seq analysis identified upregulation of AP1 transcriptional factors (TFs), such as Jun and Fos, under high collagen and stiff ECM conditions. Pharmacological inhibition of AP-1 or genetic deletion of AP1 TFs reduces collagen and ECM stiffness induced BaSC multipotency, demonstrating the key role of AP-1 activation to promote the multipotent transcriptional program in response to Collagen signaling and high ECM stiffness.
Our findings demonstrate a common mechanism mediated by Collagen/integrin β1/FAK/AP-1 signaling axis by which collagen and ECM stiffness regulate SC multipotency in the mammary gland and prostate, providing new insights into the mechanisms regulating stem cell plasticity in response to mechanical cues.
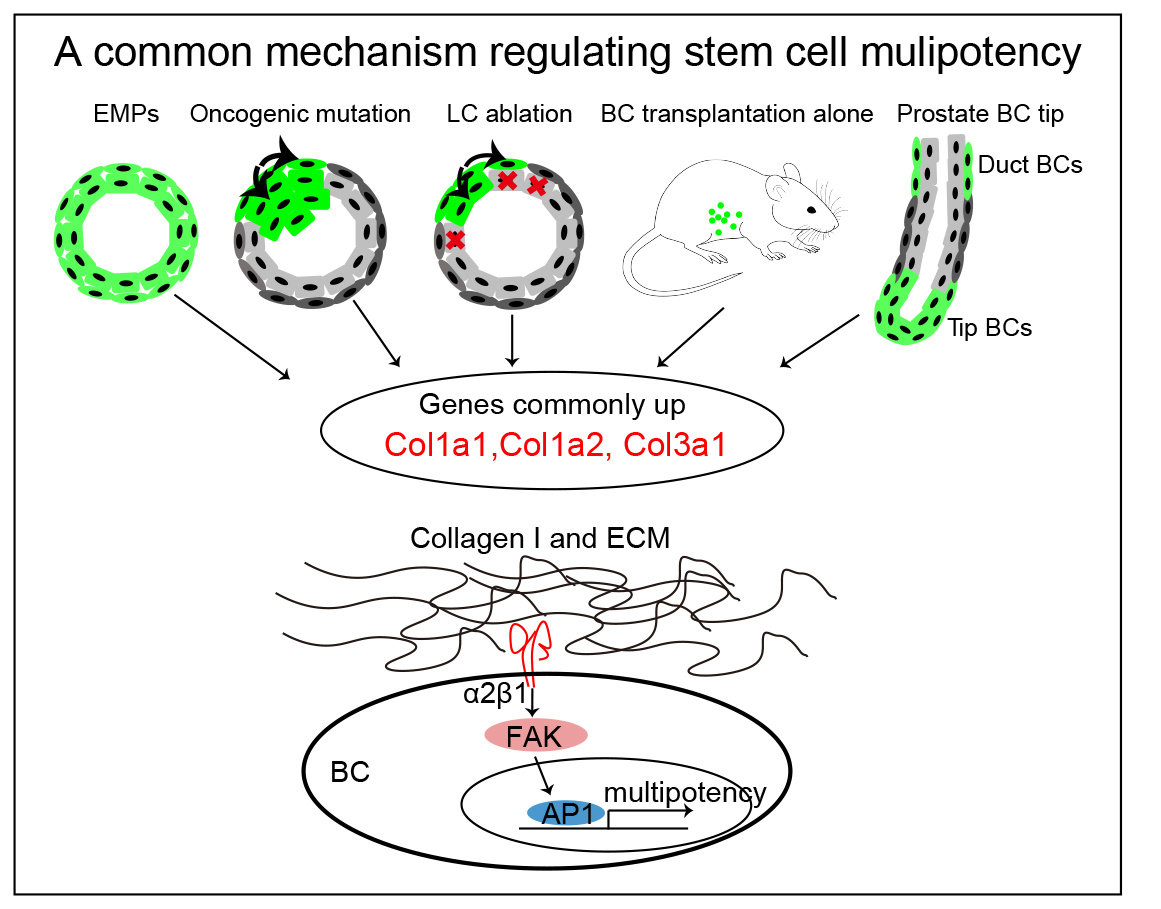
Fig. A common mechanism regulating stem cell multipotency in glandular epithelial. Genes up-regulated in EMPs (embryonic multipotent progenitors), in transplanted BCs, following PIK3CA oncogenic mutation, in BCs following LC ablation and in prostate BC tip reveals the common upregulation of Col1a1, Col1a2, and Col3a1b across all the conditions associated with BaSC multipotency in these two tissues. Our mechanistic studies reveal the key role of β1 integrin/FAK/AP-1 axis in the regulation of BaSC multipotency in glandular epithelia in response to Col1 signaling and ECM stiffness.
References
1 Wuidart, A. et al. Early lineage segregation of multipotent embryonic mammary gland progenitors. Nat Cell Biol 20, 666-676, doi:10.1038/s41556-018-0095-2 (2018).
2 Van Keymeulen, A. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189-193, doi:10.1038/nature10573 (2011).
3 Van Keymeulen, A. et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 525, 119-123, doi:10.1038/nature14665 (2015).
4 Centonze, A. et al. Heterotypic cell–cell communication regulates glandular stem cell multipotency. Nature 584, 608-613, doi:10.1038/s41586-020-2632-y (2020).
5 Tika, E., Ousset, M., Dannau, A. & Blanpain, C. Spatiotemporal regulation of multipotency during prostate development. Development 146, doi:10.1242/dev.180224 (2019).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in