A crystalline aluminum–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media
Published in Chemistry
Our group has been investigating α-substituted phosphorus ylides with the general formula Ph3PC(R1)X (where R1 = Ar, alkyl; X = BR2, SiR2Cl, GeR2Cl) for quite some time.1-4 Having successfully functionalized these ylides with boryl- (α-BCPs) and the isoelectronic cationic silyl and germyl substituents (α-SiCPs, α-GeCPs), it was of course interesting to synthesize the aluminum analogs. It quickly became apparent that the α-AlCPs were not accessible via trans-ylidation, as were the boryl- and group 14 substituted ylides. Numerous attempts over several years to metalate the nonstabilized ylides Ph3PC(R1)H in the α-position with alkali metals, magnesium, or zinc for use in salt metatheses or transmetallation also failed. However, we frequently observed the formation of the ylides (2-M-C6H4-)Ph2PCR12 (R1 = alkyl, H; M = alkali metal) metallated in ortho position of one phenyl moiety (Scheme 1).
This observation was the starting point for this project. We asked ourselves if it is possible to introduce an aluminum function in ortho position on a phosphorus ylide by simple salt metathesis. Indeed, it quickly turned out that the reaction of the lithiated ylide (2-Li-C6H4)Ph2PC(Me)2 1 with t-Bu2AlX (X = Cl, Br) was a good access to the aluminum functionalized ylide (o-AlCP) (2-{Alt-Bu2}-C6H4)Ph2PC(Me)2 2 (Scheme 1).
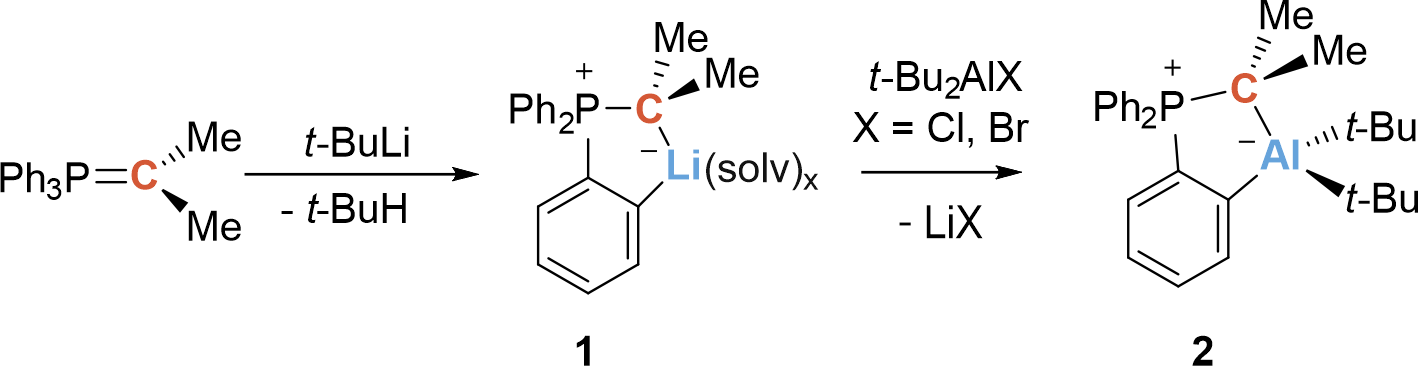
Scheme 1. Synthesis of the aluminum–carbon-based ambiphile 2.
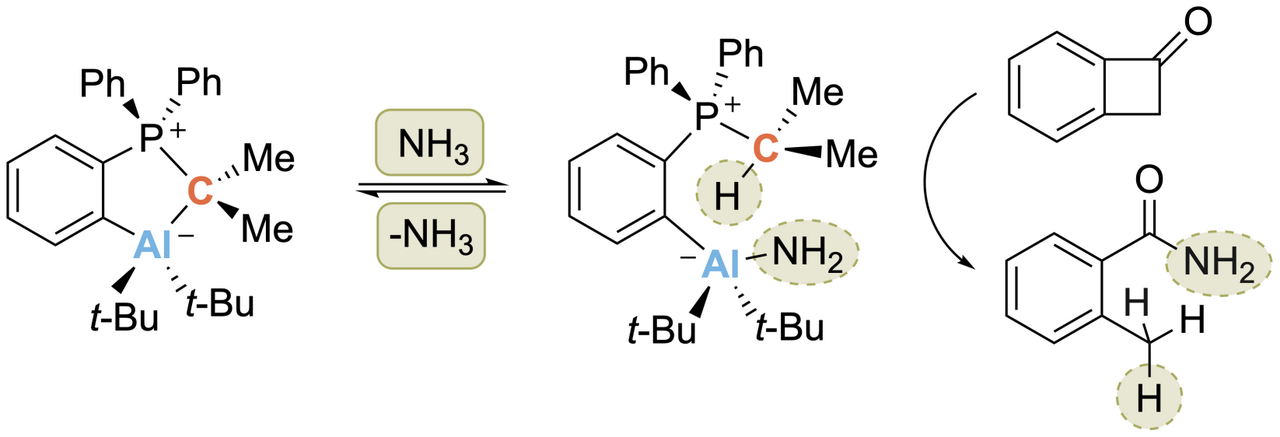
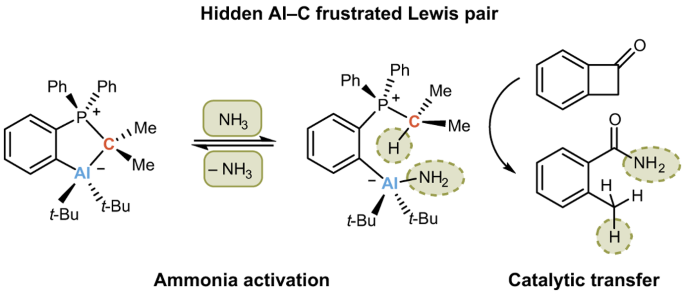
When the first crystal structure was measured, and I discussed the structure of 2 with my supervisor, the fact that it was an intramolecularly saturated system was not very inspiring at first: "To be honest, I do not expect any reactivity for this compound" was his first comment. I did not let up and tirelessly investigated the reactivity of the five-membered ring in detail. In particular, whether the CYlid-aluminum bond would react under ring-opening. The first NMR experiments were really sobering until I received the NMR spectrum of the reaction with ammonia. Contrary to expectation, it showed that the Al fragment remained bound to the ylide scaffold and that a new species was selectively formed. Quickly, the solution from the NMR tube was transferred into a vial in the glovebox to evaporate the solvent. Back to the lab the next day, I found a large colorless block at the bottom of the vial. Hooray! Crystals! It quickly turned out that this was the starting material, the closed five-membered metallacycle 2. Initial disappointment set in. But thinking about it for a moment… Did this mean that the reaction with ammonia was reversible? To investigate this further, I did a series of NMR experiments and indeed, the reaction is reversible at room temperature and ambient pressure (Scheme 2)! Now, we were able to assign the o-AlCPs to the class of compound called “hidden frustrated Lewis pairs”. A Lewis acid-base adduct that reacts like a frustrated Lewis pair under bond cleavage between acid and base.
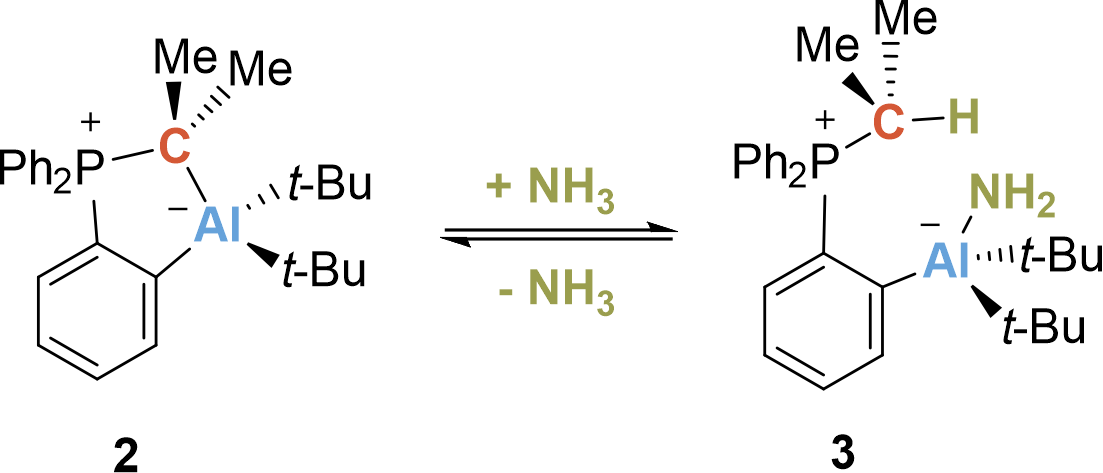
Scheme 2. Reversible N–H activation of ammonia.
After an extensive literature search on the reversible cleavage of the N-H bonds in NH3, it turned out that only some months earlier the first example of such reactivity on main group element compounds had been shown.5 Highly motivated and inspired by these findings, I made first studies on the transfer of ammonia to organic unsaturated substrates. It quickly became apparent that both nonactivated and activated C=C double bonds were no suitable substrates. Instead of hydroamination of the double bond, maleimides I and II reacted under ring opening to give the linear products, which we found in the course of the project formed oligomers (Scheme 3 a). Thus, strained rings with an electrophilic carbon atom came into our focus. The benzocyclobutenones IV and V showed full conversion and selective reaction to the corresponding carboxylic acid amides (Scheme 3 c) under catalytic conditions (RT, benzene, 20 mol% 2, 1.1 bar NH3). As was almost to be expected, one reviewer asked for more possible substrates for the transfer of the ammonia.
– At this point I would like to thank Cameron Jones and the other two reviewers for the persistent and constructive revision. –
So, in total I tested 31 substrates and we were able to show the catalytic activity nicely using the alkylation of NH3 with benzylbromide VI as an example. Without 2, the conversion of the reaction after 84 h is 7 %. In the presence of 2 the conversion is 49 % during the same time. Benzylamine VIa is formed, which reacts further with benzylbromide to give tribenzylamine VIb in a ratio of 1.0:1.2 after complete consumption of VI (Scheme 3 c).
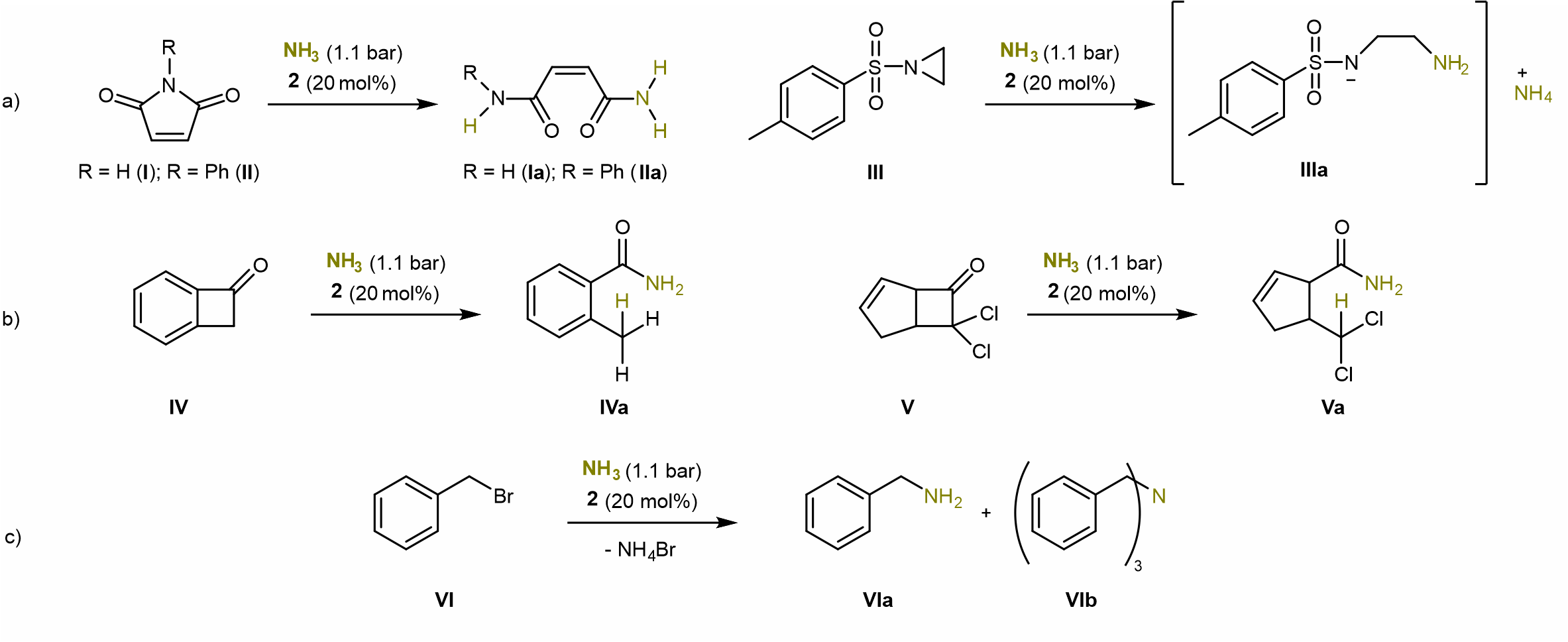
Scheme 3. Catalytic ammonia transfer reactions in benzene at RT (p = 1.1 bar; 20 mol% compound 2).
This work represents an important step in the difficult activation and transfer of non-aqueous ammonia and opens a promising field of research. Follow-up studies on similar aluminum- and gallium-based ambiphiles, with phosphorus ylides as Lewis-basic components, are part of current studies in our laboratories. At the end, I would like to thank everyone who has participated in this project for their perseverance, encouragement and support during the ups and downs of this project.
1. Radius, M. & Breher, F. α-Borylated Phosphorus Ylides (α-BCPs): Electronic Frustration within a C−B π-Bond Arising from the Competition for a Lone Pair of Electrons. Chem. Eur. J. 24, 15744-15749, doi:https://doi.org/10.1002/chem.201803823 (2018).
2. Radius, M., Sattler, E., Berberich, H. & Breher, F. Reactivity of a Sterically Unencumbered α-Borylated Phosphorus Ylide towards Small Molecules. Chem. Eur. J. 25, 12206-12213, doi:https://doi.org/10.1002/chem.201902681 (2019).
3. Krämer, F., Radius, M., Berberich, H., Fernández, I. & Breher, F. A neutral, acyclic, borataalkene-like ligand for group 11 metals: L- and Z-type ligands side by side. Chem. Commun. 58, 3905-3908, doi:10.1039/D2CC00199C (2022).
4. Krämer, F., Radius, M., Hinz, A., Dilanas, M. E. A. & Breher, F. Accessing Cationic α-Silylated and α-Germylated Phosphorus Ylides. Chem. Eur. J. 28, e202103974, doi:https://doi.org/10.1002/chem.202103974 (2022).
5. Abbenseth, J., Townrow, O. P. E. & Goicoechea, J. M. Thermoneutral N−H Bond Activation of Ammonia by a Geometrically Constrained Phosphine. Angew. Chem. Int. Ed. 60, 23625-23629, doi:https://doi.org/10.1002/anie.202111017 (2021).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in