A high-resolution large-area detector for quality assurance in radiotherapy
Published in Healthcare & Nursing and Physics
Hadron therapy, an advanced cancer treatment, utilizes cyclotrons and synchrotrons to accelerate protons and carbon ions to irradiate tumors by delivering a highly conformal dose distribution.
Precise dose calculation and verification are crucial, ensuring accurate delivery to target areas while sparing nearby healthy tissue. This is in turn guaranteed by appropriate Quality Assurance (QA) protocols and a proper set of detectors for measuring the beam parameters, in particular the beam position and the delivered dose distribution.
How can we provide higher quality treatments, better patient outcomes and reduce healthcare costs?
One possible way is the improvement of QA by developing an advanced detector system to improve current quality control protocols and dosimetry procedures. Improvements towards an all-in-one system offering precise and real-time measurements with sub-millimeter spatial resolution and uniform response to the beam energy are feasible today. We aimed at the inclusion of all necessary information in the QA and treatment plan verification to enhance the quality of patient treatment.
Our ultimate goal was to build a detector that combines a better performance than current commercial devices with significant time reduction at every step of the Machine and Patient QA chain, leading to a more efficient QA workflow. Hence, the purpose of this work was to develop a novel large area detector based on GEM technology, providing 2D dosimetric imaging of ion beams with sub-millimeter spatial resolution. A large sensitive area is required to cover the typical radiation field size and evaluate the dose distribution in the entire treatment area.
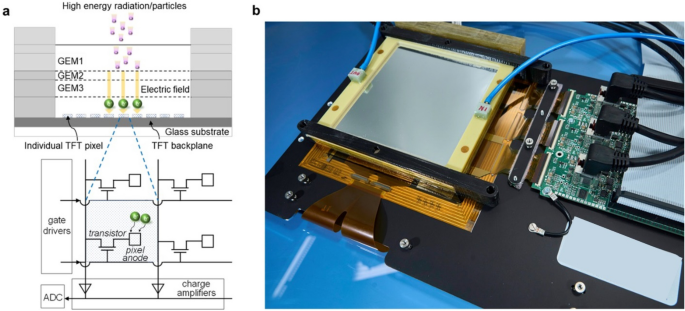
In this paper we present for the first time the applicability of flat panel TFT technology as GEM detector readout, to obtain large area beam imaging with high spatial resolution at high frame rates (Figure 1).

Figure 1- High resolution GEM-TFT radiation detector. a. Schematic of the detector, consisting of a TFT backplane array and a triple-GEM frontplane. The magnification shows the TFT pixel layout, together with the drive and read-out circuit diagram. b. Photograph of the detector integrated with the read-out electronics.
Our primary objective was to investigate the feasibility of this innovative technology, which holds the promise of scalability within the semiconductor industry. This technology fusion seeks to capitalize on the strengths of gaseous detectors, such as their minimal material requirements and potential use of tissue-equivalent gases, while surmounting the limitations of ionization chambers regarding pixel size and the number of detectors in a matrix.
The investigation followed several paths at different points in time, leading to the development of several prototypes, which we briefly describe below.
-
- The first prototype combined a triple GEM and a highly pixelated readout based on a matrix of organic photodiodes coated on an oxide TFT backplane [1]. GEM were invented at CERN by F. Sauli in 1996; a typical GEM foil is made of a 50 µm thick insulating Kapton layer coated with conductive metal on both sides. Holes etched in the foil and an applied voltage create high electric fields within the holes. When electrons pass through these holes, avalanche multiplication occurs, producing secondary electrons per primary electron, depending on factors like gas density, gas mixture, and electric field. The triple GEM configuration used for all the prototypes can achieve gains ranging from 100 to 10,000. The first version with optical readout proved to have a limited spatial resolution.
- To improve it, we built a new version of the detector with a reduced gap between the last GEM foil and the readout, which still did not meet the requirements of the sub-millimeter spatial resolution [2]. Since the distance between the last GEM and the readout could not be further reduced due to mechanical reasons, a sub-millimeter spatial resolution is hardly reachable with an optical readout without the introduction of lenses.
- As the isotropic emission of the scintillation light was identified as the main limitation, a charge readout was then investigated. In this design, secondary electrons produced in the GEMs and guided by electric fields are directly detected by the readout. We demonstrated that the novel detector with charge readout can measure secondary electrons produced by the triple-GEM structure under high intensity beams of various types and sources of high-energy radiation, i.e., X-rays, protons, and carbon ion beams. We demonstrated that it is possible to have a large-area beam imaging with both high spatial resolution and compact set-up. The sub-millimeter spatial resolution is possible by the direct readout of the charge, the directionality of the charges from the GEM and the high pixel density of the TFT array.
The detector was designed and built at CERN, partly financed by the ATTRACT project funded by the EC under Grant Agreement 777222, which allowed the development of the first TFT prototype in collaboration with TNO/Holst Center, Eindhoven, The Netherlands. The research work was conducted in the context of a PhD in Medical Applications of Particle Physics at the Laboratory for High Energy Physics (LHEP) and Albert Einstein Center for Fundamental Physics (AEC) of the University of Bern, and was co-supported by a grant from FCT with reference SFRH/BEST/142965/2018. Additionally, this project has been co-funded by the CERN Budget for Knowledge Transfer to Medical Applications. View the video below to explore 'The Idea in 60 Seconds':
The initial envisaged application for the detector focused on hadron therapy. Nevertheless, it is noteworthy that, due to the lower cost and larger accessibility, the prevailing global trend in cancer treatment predominantly favors photon-based therapies, which annually treats around 7 million patients, over hadron therapy. This observation underscores the imperative for continued advancements for both treatment modalities.
The detector showed a linear response to proton intensities over two orders of magnitude, spanning the typical doses used in hadron therapy. Moreover, the detector showed relatively low radiation damage, that can be compensated by virtue of the two separate gate electrodes in the TFT.
The first characterization tests were performed at the Calibration Laboratory of CERN's Radiation Protection group. Measurements with accelerated protons were performed at the medical cyclotron laboratory located at the SWAN-Haus at the Bern University Hospital (Inselspital). The prototype was tested at a commercial TrueBeam Linac by Varian, utilizing a 6 MV FF photon beam, and was also tested at CNAO in Italy with clinical proton and carbon ion beams.
The performance tests affirm the instrument's capability to detect a range of energies, encompassing low-energy (40 kVp X-rays) and high-energy (6 MeV) photons, as well as protons and carbon ions of clinical energies.
In conclusion, this work paves the way for the realization of a future commercial detector employing this technology. Such advancements hold promise for diverse applications in medical physics, contributing significantly to the enhancement of QA across various human-related beam applications. Moreover, it serves as the starting point and motivation for future development of even more refined device architectures that further boost the performance of quality assurance in cancer radiation therapy.
References:
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in