A human-on-chip for drug testing
Published in Bioengineering & Biotechnology

The challenge
Long timelines (> 10 years), high costs (close to $3 billion), and low success rates (only 1 out of 5,000 drugs gets to patients) plague the development of new drugs. Moreover, even the top 10 best-selling drugs work for fewer than 25% of patients.
Organ-on-chips enable the culture of engineered human tissues and are increasingly successful in modeling human physiological functions. They have been used to study the efficacy, safety, and pharmacological profiles of drugs since their initial development in the early 2010s.
In the body, each organ maintains its own environment, while at the same time interacting with other organs via circulating cells and bioactive factors in the blood circulation. The blood vessels are lined by endothelial cells, allowing selective transport of cells and molecules in response to biological signals, such as the signals generated by tissue injury which attract immune cells. The individual organ environments and organ communication are critical for tissue homeostasis in health and disease, and they make it challenging to accurately model conditions that involve several organs, such as cancer, fibrosis, inflammation, and infection.
While significant advancements have been made in the design and application of organ-on-chips, there has been a lingering need to enable the co-culture of multiple organ systems for evaluating organ responses at a systemic level. The current approaches to culturing multiple connected tissues use the same medium for the entire system, containing a mixture of all required regulatory factors, rather than exposing each tissue to its own regulatory factors. Under these conditions, the connected tissues quickly lose their organ-specific features, limiting the ability to model human physiology.
Inspired by the enormous potential of organ-on-chips to improve the development of new drugs, our group set out to develop an organ-on-chip able to fully recapitulate human physiology in vitro with two requirements in mind:
- Provide each organ its own environment (to maintain each specific tissue phenotype for weeks-to-months as required for biological and biomedical studies), and
- Link organ modules to each other to enable their communication (required for modeling conditions that involve more than one organ system).
It takes a village
We started by developing a chip with heart, liver, and vasculature, nicknamed the HeLiVa platform. After ten years, hundreds of experiments, and many great ideas and serial prototypes, we finally developed this chip that captures the biology of tissue and organ interactions in the body. At the Laboratory of Stem Cells and Tissue Engineering at Columbia University led by Prof. Gordana Vunjak-Novakovic, we assembled an inter-disciplinary team with the necessary expertise and resources to tackle this challenge. As in all our research projects, we worked together to develop several of the engineered tissues (liver, heart, bone, skin, and vasculature) and the culture systems where they are matured and integrated. Prof. Angela M. Christiano and her team at Columbia University developed the mature human skin, while Prof. Karen K. Hirschi (University of Virginia) and Prof. Cristopher S. Chen (Boston University) brought to the team their expertise in vascular biology and engineering. Prof. Andrea Califano and his systems biology team at Columbia University analyzed the miRNA sequencing data, crucial for the identification of biomarkers of cardiotoxicity, and Prof. Rajesh Soni helped in the proteomics analysis. The team at CFD Research Corporation was instrumental in the development of the mathematical model of the multi-organ chip. This multi-year study is an example of how a team of researchers with different backgrounds can work toward a shared purpose with great success.
Our approach: Connect mature engineered tissues via vascular flow while maintaining their individual tissue-specific niches
Our goal was to develop a multi-organ chip consisting of engineered human tissues linked by vascular flow. We envisioned the possibility of creating a patient-on-chip, a personalized chip with tissues derived from cells donated from a single patient. To that end, we used human induced pluripotent stem cells and differentiated them into the cells needed to bioengineer various tissues. Because tissues engineered from stem cells are inherently immature and must be induced to maturation in order to present an adult-like phenotype, we developed and matured each of the tissues separately, always with the mindset that cells are the true tissue engineers, and connected them in a way that would preserve their individual biological fidelity.
As detailed below, we accomplished this by taking inspiration from how the human body overcomes this same challenge: combining the maintenance of individual environments (tissue modules, each with its own medium composition, tissue matrix, and physical regulatory factors) with tissue-tissue communication (across a selectively permeable endothelial barrier and via vascular circulation). We also introduced immune cells into vascular circulation because of their important role in tissue responses to injury, disease, and treatment.
Over several years, we developed numerous designs until we settled with the design presented in this manuscript (Fig. 1). Our goal was to recapitulate human physiology by emulating the biological functions of a subset of interconnected organs in a configurable setting designed for high-content screening. This design enables us to connect the tissues in any desired configuration and number, and to collect functional real-time readouts. The chip is uniquely designed for studies of systemic conditions of injury or disease, allowing us to maintain the biological properties of patient-specific engineered human tissues along with their communication.
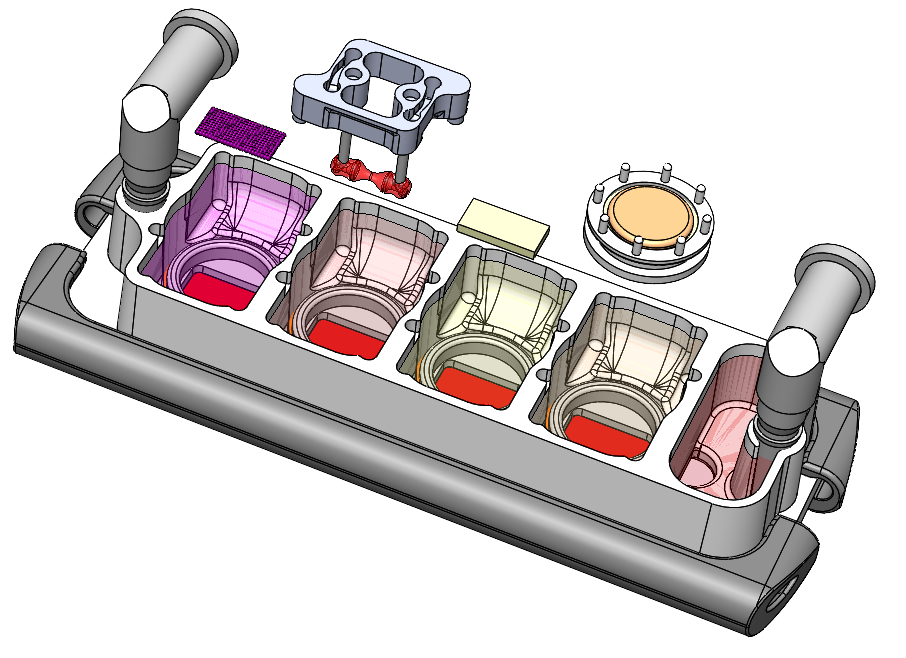
Fig. 1. Multi-organ chip with four human engineered tissues (liver, heart, bone, and skin) connected by vascular flow with the endothelial barriers separating tissue spaces and vascular spaces.
Did it work?
The multi-organ chip enabled us to connect tissues for long periods of time (four weeks) without sacrificing their individual biological fidelity, a result not achieved when they were cultured without an endothelial barrier. We were able to validate that the linked organs communicated with one another by tracking fluorescently labelled exosomes generated by the heart as they travelled to adjacent tissues. We further showed tissue specific immune responses by injuring one tissue and showing that the subsequent immune response to injury was only present in the injured tissue.
We were pleased to see that the pharmacodynamics (tissue responses to a drug) of doxorubicin, the broadly used anticancer drug we studied, matched closely with the clinical data. We simultaneously developed a novel computational model of the chip for mathematical simulations of the drug's pharmacokinetics (distribution and metabolism in the tissues and vascular flow). This in silico model correctly predicted doxorubicin’s metabolism into doxorubicinol and its diffusion into the chip. Most notably, the multi-organ chip also identified cardiotoxicity and cardiomyopathy biomarkers, a result we were very excited about.
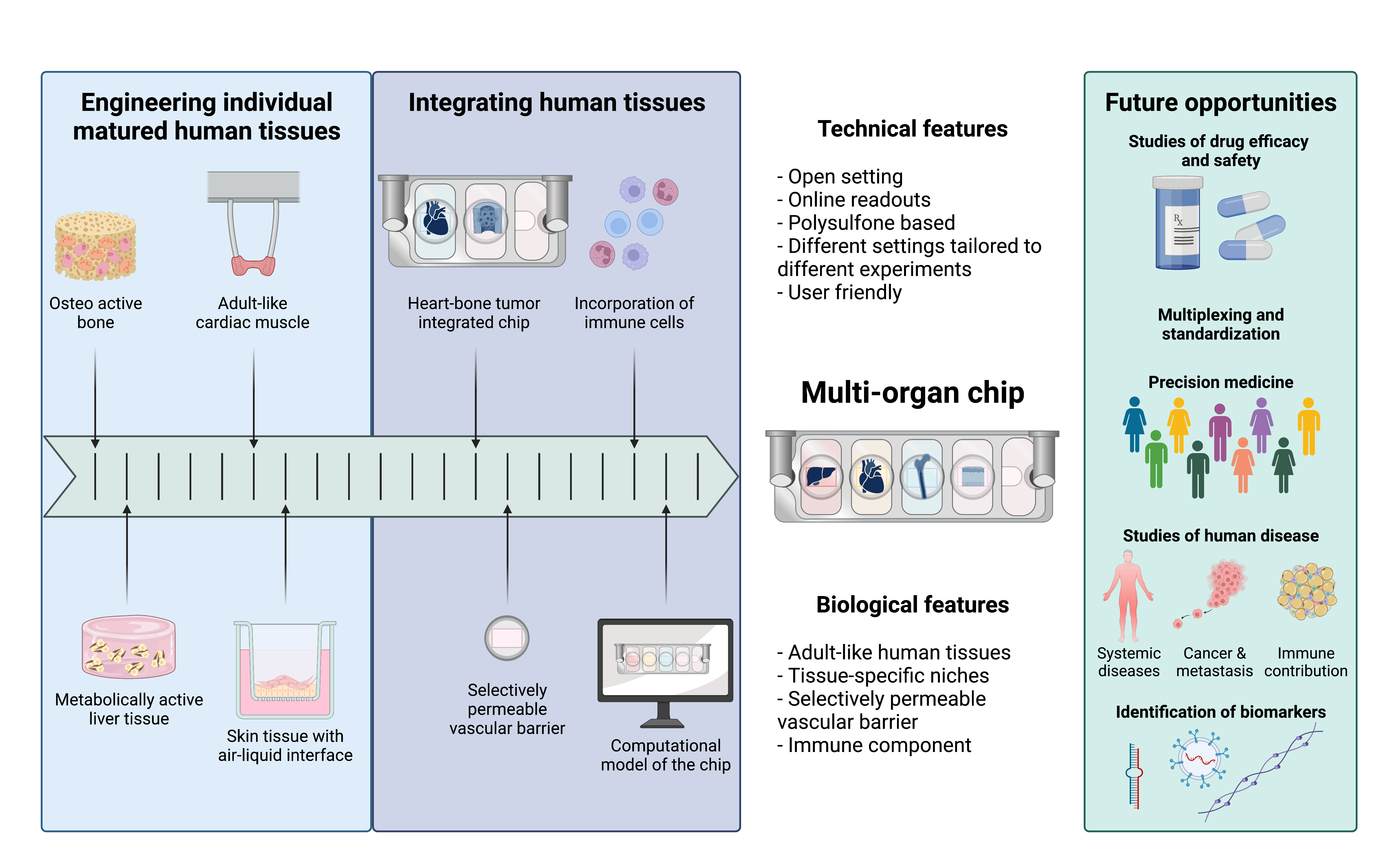
Fig. 2. From engineering individual matured human tissues to an integrated multi-organ chip for precision medicine and pharmacological studies. Created with BioRender.com.
Overall, our multi-organ chip model enables the maintenance of tissue-specific niches, necessary for the physiological tissue functions and the recapitulation of systemic effects. This chip, in combination with computational methodology, provides an improved model for future studies of pharmacokinetics and pharmacodynamics by faithfully representing individual tissue maturation together with tissue-tissue communication, overcoming many of the challenges of other traditional model systems (Fig. 2). We are excited about the potential use of our chip, which we are currently using to study breast and prostate cancer metastasis, effects of radiation, effects of ischemia, as well as the pharmacokinetics and pharmacodynamics of other drugs.
Text and figures by Diogo Teles, Kacey Ronaldson-Bouchard, and Keith Yeager.
Our paper: Ronaldson-Bouchard, K., Teles, D., Yeager, K. et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng 6, 351-371 (2022). https://doi.org/10.1038/s41551-022-00882-6
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in