One-day sterility testing for the age of biopharmaceuticals
Published in Bioengineering & Biotechnology and Biomedical Research

The Shadow Behind the Advancement of Biopharmaceuticals
Can we justify administering therapies to patients before confirming sterility?
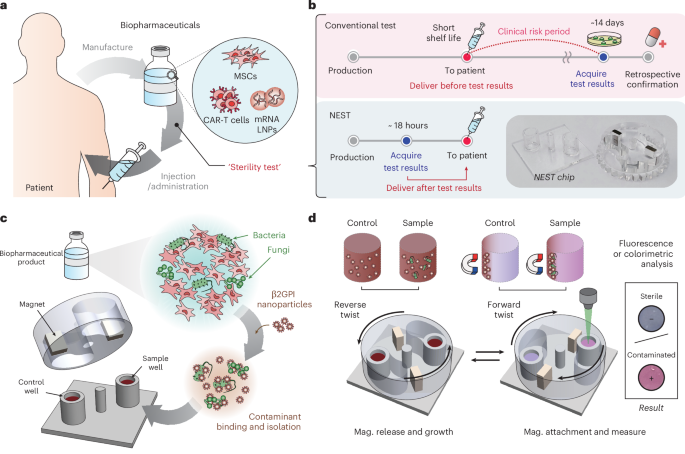
Medicines are fundamentally expected to be sterile, as contamination can immediately turn treatment into danger. Since the 1930s, drug sterility has been regulated by the 14-day culture test, a method that waits until contaminants, if present, proliferate to detectable turbidity levels. For decades, this standard posed no issue for conventional (chemical) drugs, as they typically undergo terminal sterilization prior to their release. However, recent advances in tissue and genetic engineering have brought forward a new generation of living, patient-derived therapies such as CAR-T cells and stem cell products. These biopharmaceuticals cannot undergo terminal sterilization, often expire within only a few days, and must be administered urgently to critically ill patients, making the 14-day test fundamentally incompatible with real-world clinical practice. Just as biopharmaceuticals have advanced rapidly, sterility testing should have evolved accordingly, yet it has remained unchanged.
The Call That Launched Our Journey
Prof. Eun Ju Lee of Seoul National University Hospital’s Advanced Cell & Gene Therapy Center has long recognized that legal and regulatory frameworks lag far behind the rapid progress of advanced medicines. Until 2019, South Korea had no dedicated law governing advanced biopharmaceuticals.
This gap became evident during the Kolon Invossa incident in 2019, when it was revealed that the therapy contained HEK293 human embryonic kidney–derived cells rather than the approved chondrocytes. The revelation led to product withdrawal and clinical trial suspensions, exposing both weaknesses in quality control and the absence of a regulatory framework. In the aftermath, Korea enacted the Act on the Safety and Support of Advanced Regenerative Medicine and Advanced Biopharmaceuticals in 2019, which came into effect in 2020 as the nation’s first dedicated legal system for cell and gene therapies.
Following the Invossa case, regulatory authorities began requiring separate QC approvals for each product type. Around the same time, Seoul National University Hospital was establishing Korea’s first GMP facility for CAR-T cell manufacturing. While setting operational standards, Prof. Lee confronted a critical dilemma: for patient-derived cell therapies, it was practically impossible to wait for sterility results before infusion. Despite repeated discussions, no validated rapid sterility test was available, and regulators and clinicians agreed on an interim solution in which in-process samples collected three days earlier were incubated for another three days. If no contamination was detected, the therapy proceeded to infusion. This policy allowed timely administration of the therapy, yet the sterility of the final product remained unverified. “It was unsettling to deliver a therapy without confirmed sterility,” Prof. Lee later reflected.
Even today, under USP guidelines, a risk-based approach remains common worldwide, in which therapies are infused first and sterility is confirmed only afterward. Convinced that such a practice should not continue, Prof. Lee began seeking a technical alternative that might ultimately enable regulatory change. This search led her to our engineering team, which was then developing ultra-rapid antibiotic susceptibility testing (uRAST, Nature, 2024). Seeing how quickly microbes could be identified, she thought, “If pathogens can be detected this fast, perhaps sterility testing could be reimagined as well.” That single call became the starting point of this study.
Why Rapid Sterility Testing Proved So Difficult
At first, we expected that adapting existing microbial detection technologies to sterility testing would not be so difficult, since we were already familiar with many methods. Yet despite decades of development, none had translated into the sterility testing field, and there were clear reasons why.
- Diversity of biopharmaceuticals: Products range from proteins and antibodies to live cells. In particular, therapies containing around 106 therapeutic cells per mL, whose physical and metabolic properties closely resemble those of microbes, made it extremely difficult to distinguish rare microbial contaminants from the overwhelming background of host cells.
- Unpredictable contaminants: Potential contaminants were unpredictable, ranging from bacteria to fungi and encompassing both aerobes and anaerobes.
- Extreme sensitivity requirements: The assay had to be sensitive enough to detect 1 CFU.
Above all, proving the absence of microbes within hours was far more challenging than performing the subsequent diagnostic steps after confirming their presence. Furthermore, because our goal was not merely to build a research tool but to develop a technology that could ultimately inform regulatory practice, we had to seek for an approach that was simple, inexpensive, automatable, and compatible with existing regulatory frameworks.
The Breakthrough
Nevertheless, we succeeded in developing NEST (Nanoparticle-based Enrichment and rapid Sterility Test), a system capable of completing sterility testing within a single day. In our study, NEST detected microbes at concentrations as low as 1 CFU/mL, with detection times ranging from 5 to 18 hours depending on the species. On average, this was about 60 hours faster than conventional assays, all while maintaining high reliability. Importantly, we demonstrated that NEST could be applied across diverse advanced biopharmaceuticals, including clinical-grade stem cells, CAR-T therapies, and drug delivery systems, showing that its performance was independent of product composition. The system was further validated under clinically relevant scenarios, such as anaerobic organisms, polymicrobial mixtures, and resistant strains, and consistently produced reliable results. All of these capabilities were achieved through a simple and practical testing workflow.
What’s Next
We have always envisioned engineering research not only as a driver of scientific progress but also as a means to transform the real world. Today, we are advancing automation and preparing clinical studies in close collaboration with hospitals. Just as regulatory frameworks evolve alongside technological innovation, we believe that in the era of advanced biopharmaceuticals such change is both essential and inevitable. In the past, regulations persisted largely because no alternative technologies were available. Now that NEST offers such a solution, it sets the stage for future regulatory discussions and for ensuring safe administration in line with the advancement of biopharmaceuticals. While adoption may first occur in therapies requiring urgent administration, the technology holds the potential to extend across the broader landscape of biopharmaceuticals.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in