A new opportunity for Aurora A inhibitors: improving the efficacy of anti-EGFR therapies via targeting YAP1
Published in Cancer
Background
Aurora A, a key regulator of cell division, is often overexpressed in tumors, making its inhibitors promising for cancer treatment. However, their failure in clinical trials underscores the need for better patient selection (1,2). Recent research has shown that AURKA can phosphorylate YAP1's Ser397 (3), enhancing its stability and transcriptional regulation and suggesting a potential role for this kinase beyond mitotic functions.
YAP1 has been implicated in primary resistance to cetuximab in metastatic CRC (4), affecting 40% of patients. It highlitghts the clinical need for novel predictive biomarkers and combined therapies to improve cetuximab efficacy. Unfortunately, YAP1 is considered an undruggable target (5), prompting investigation into the joint role of YAP1 and AURKA as drivers of primary resistance to cetuximab.
Main Findings
Following a bioinformatic analysis suggesting increased YAP1 activation, but no expression, in cetuximab-resistant CRC cell lines, we observed YAP1 hyperphosphorylation at Ser397 in some of these resistant lines. This phosphorylation, described to be catalyzed AURKA, correlated with elevated expression of YAP1 transcriptional targets, supporting its activating role. Treatment with the AURKA inhibitor alisertib disrupted YAP1 phosphorylation at Ser397, thereby restoring cetuximab efficacy. These results were further validated using an in vivo model, where the CRC tumor displaying the highest YAP1 phosphorylation level was selected for a patient-derived xenograft (PDX) model. As expected, this tumor exhibited resistance to cetuximab monotherapy but regained sensitivity upon low-dose alisertib treatment, contingent upon suppression of YAP1 phosphorylation. Importantly, the AURKA-sensitizing effects were limited to models with YAP1 hyperphosphorylation, highlighting the specificity of AURKA inhibition in exerting its effect through YAP1. This regulation of YAP1 by AURKA was independent of the Hippo pathway, as evidenced by unchanged phosphorylation levels of the main Hippo kinases, LATS1, and MOB1, after AURKA inhibition. These findings suggest novel non-mitotic functions of AURKA and novel checkpoints for regulating YAP1 activity beyond the classical Hippo kinase cascade.
YAP1 has been implicated in mediating stemness and drug resistance in CRC (6). Thus, we aimed to discern if the acquisition of a stem-like phenotype differs in cetuximab-resistant tumors compared to sensitive ones. Our findings revealed that resistant CRC cell lines overexpressed numerous stemness-related gene signatures. Additionally, in vitro phenotypic validation demonstrated heightened stem-cell properties in the cetuximab-resistant cell lines. By ransducing the resistant CRC cells with a dominant-negative mutant of YAP1 lacking the Ser397 residue (S397A) we revealed that YAP1 is unable to induce stemness when it cannot be phosphorylated by AURKA, confirming the activating nature of Ser397 phosphorylation and its specific functions in driving the cancer-stem cell phenotypes. Furthermore, AURKA inhibitors suppressed stem-like properties both in vitro and in vivo, underscoring the AURKA/YAP1 axis as a driver of stemness and drug resistance in CRC.
Cancer stem cells play a crucial role in driving therapeutic resistance in CRC (7), with YAP1 emerging as a key driver of this phenomenon. However, the specific involvement of cancer stem cells in mediating resistance to cetuximab in CRC remains largely unexplored. In our study, we elucidate the role of AURKA in finely modulating YAP1 activity by phosphorylating its Ser397 residue independently of the Hippo pathway. This phosphorylation stabilizes YAP1 and enhances its transcriptional activity, triggering a cell reprogramming towards stem cell traits. Inhibition of AURKA not only suppresed the stem-like properties of resistant cells, but also restored the cetuximab efficacy both in vivo and in vitro (Figure 1).
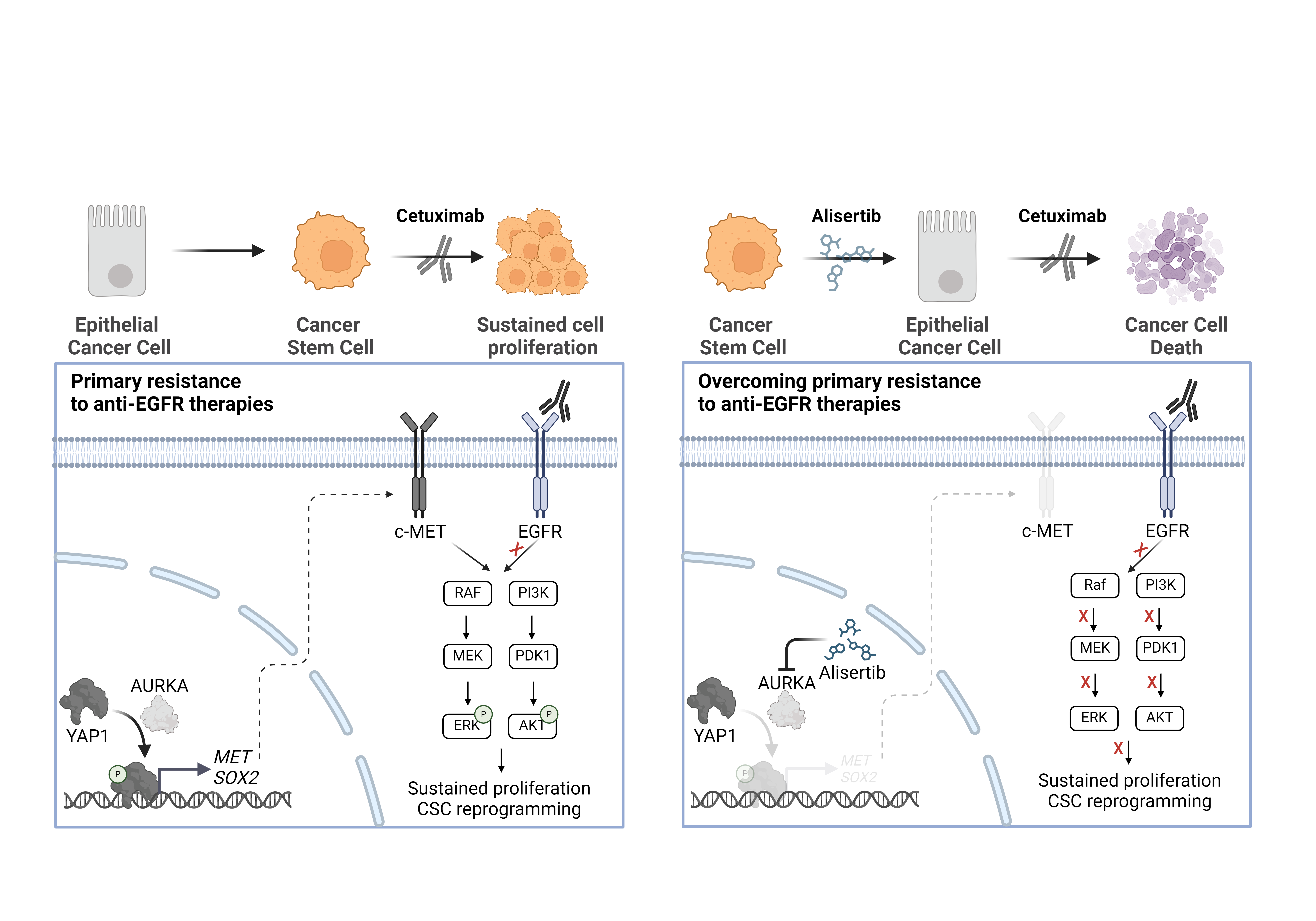
Notably, all the reported therapeutic effects were achieved using the lowest possible dosage of alisertib. While in vitro assays utilized doses resulting in less than 30% cell death, doses administered to the PDX model were three to four times lower than the standard. Given that any new therapy for metastatic CRC combined with cetuximab would be integrated into the standard of care FOLFOX/FOLFIRI, minimizing side effects is crucial to ensuring its clinical viability.
Take-home messages.
Our discoveries present a promising therapeutic strategy centered on the AURKA/YAP1 axis to eliminate drug-resistant cancer stem cells. This approach holds promise for demonstrating favorable tolerability and efficacy in enhancing responsiveness to cetuximab. Such advancements have the potential to broaden treatment options and enhance the likelihood of survival for a considerable number of patients.
- Manfredi MG, Ecsedy JA, Chakravarty A, Silverman L, Zhang M, Hoar KM, et al. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011 Dec 15;17(24):7614–24.
- O’Connor OA, Özcan M, Jacobsen ED, Roncero JM, Trotman J, Demeter J, et al. Randomized Phase III Study of Alisertib or Investigator’s Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma. J Clin Oncol. 2019 Mar 10;37(8):613–23.
- Chang SS, Yamaguchi H, Xia W, Lim SO, Khotskaya Y, Wu Y, et al. Aurora A kinase activates YAP signaling in triple-negative breast cancer. Oncogene. 2017 Mar 2;36(9):1265–75.
- Wu DW, Lin PL, Wang L, Huang CC, Lee H. The YAP1/SIX2 axis is required for DDX3-mediated tumor aggressiveness and cetuximab resistance in KRAS-wild-type colorectal cancer. Theranostics. 2017 Feb 27;7(5):1114–32.
- Xie X, Yu T, Li X, Zhang N, Foster LJ, Peng C, et al. Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials. Sig Transduct Target Ther. 2023 Sep 6;8(1):1–71.
- ABHD5 inhibits YAP-induced c-Met overexpression and colon cancer cell stemness via suppressing YAP methylation | Nature Communications [Internet]. [cited 2024 Mar 5]. Available from: https://www.nature.com/articles/s41467-021-26967-5
- Das PK, Islam F, Lam AK. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells. 2020 Jun 3;9(6):1392.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in