A perfectly timed exit: the Lassa virus’ clever strategy to protect its spike protein
Published in Microbiology

Without its spike complex, an enveloped virus is just a genome-encoded particle, diffusing aimlessly with no means of self-replication. Yet with this protein, the virus is equipped to become a potent pathogen, capable of entering and taking over its cellular host. The spike is essentially the “eyes and ears” of the virus, and it is required to begin the infection process. What comes along with its critical role is that the spike must be stable enough to withstand the harsh conditions of the extracellular environment. Additionally, as one of the few protein complexes expressed on a virion’s surface, it is constantly under threat from the host’s immune system. This interplay between the virus and its target host cell, while under continual immune surveillance, puts selective pressure on the virus to utilize an effective infection strategy. It is thus of paramount importance to fully characterize the spike protein and understand its role within the virus’s lifecycle. Scientists have typically focused on how the spike is utilized during viral entry. However, an understudied area is how exactly the viral progeny can leave the cell and what role the spike protein plays in this process.
Once a virus has entered its host cell, released its genome into the cytoplasm, and proceeded to replicate itself, the newly formed virions are then faced with the challenge of escaping from their host. This process, known as viral egress, is essential for bringing virions into the extracellular space so they can perpetuate the infection cycle. We know that viruses use several general strategies for egress, such as budding, cellular lysis (i.e., bursting), and exocytosis (secretion from vesicles). There are, however, many creative modifications to each of these exit strategies. The most well studied is likely that of influenza, which co-expresses neuraminidase on its surface. The virus uses neuraminidase to degrade sialic acid, which is its primary host receptor, from the cell surface during budding1. Another recently discovered exit strategy that HIV and other retroviruses can utilize is to hijack the tunneling nanotube (TNT) network, which is used for intracellular communication. This strategy eliminates the need for cellular receptors and allows the virion to directly enter a neighboring cell2. In our lab, we recently came across a unique strategy that can improve the success rate of viral egress.
I’ve been studying the Lassa virus, which is a prominent member of the Arenaviridae family and is endemic throughout West Africa. This deadly pathogen infects thousands of people each year and produces “Lassa fever” - a hemorrhagic disease that can have debilitating lifelong symptoms for its survivors. The virus is primarily spread through the excreta of the Mastomys rat, but human-to-human transmission has also been documented3. Lassa expresses a spike complex on its surface, which it utilizes to bind its host receptor, α-dystroglycan (α-DG). The binding event between Lassa’s spike and α-DG effectively begins the infection process. It was previously discovered that Lassa actually binds a long string-like polysaccharide – called matriglycan6 – which extends off the surface of α-DG. This discovery7,8 begged the question: how does Lassa grab onto this sugar moiety and use it to approach the cellular membrane?
To determine how exactly the Lassa spike interacts with matriglycan - we set out to obtain the structure of the spike complex bound to its endogenous receptor using single-particle cryo-electron microscopy (cryo-EM). Previous structural studies had focused on using crystallization to determine the structures of Lassa’s spike subunits, independently, which were present in their non-native states. Additionally, it was observed that the ectodomain of the spike (the portion outside the viral membrane) disassembles when expressed independently – if no stabilizing modifications are used. We therefore assumed that to observe binding between the Lassa spike & its receptor, we would need to use the full-length native spike complex – similar to how it would be found in nature, present within the viral membrane. This was a challenging task, as it was unclear if there even is an extraction method that could preserve the integrity of the spike complex during purification.
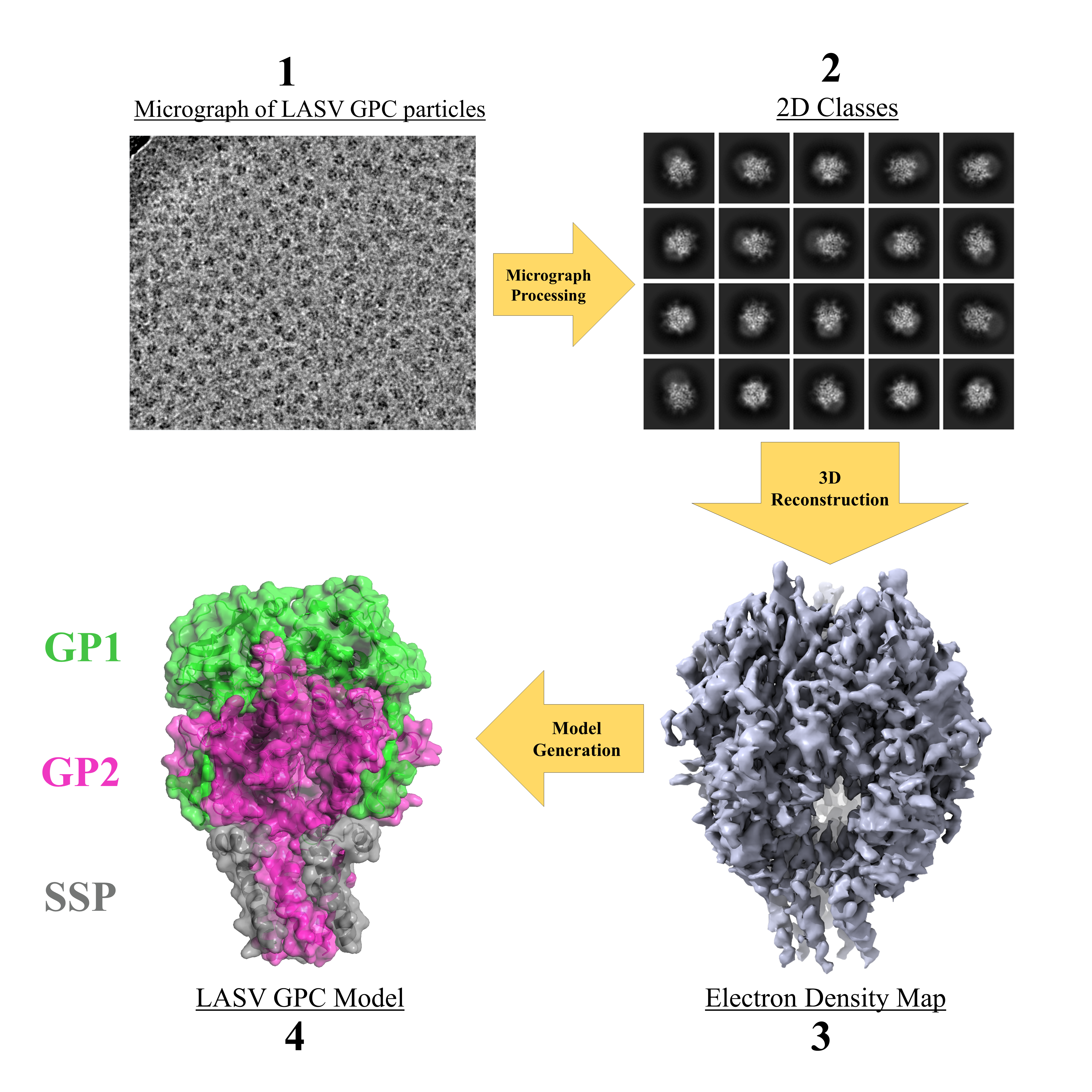
Figure 1: The steps for structural determination of purified Lassa spike complex
(LASV GPC).
Before attempting to copurify the Lassa spike & α-DG together, we first hoped to obtain the structure of Lassa’s spike alone, solubilized in a detergent micelle. After a very long optimization process – with significant trial & error – we succeeded in obtaining a 3D electron density map of the spike complex. We then built off the results of a previous study9 and successfully generated a model for this protein (Figure 1). Lassa’s spike was observed as a trimeric complex – where each monomer is composed of three subunits – GP1 (for host recognition), GP2 (for fusing the viral and host cell membranes), and the stable signal peptide (SSP). Signal peptides are common in the protein world, but they are typically cleaved off and degraded directly after the protein is transported to its desired location. Arenaviruses have an unusually long SSP that integrates into the spike complex4. The incorporation of the SSP as a spike subunit is believed to be unique to arenaviruses and has thus puzzled scientists since this discovery a couple of decades ago5. The orientation of the SSP within the viral membrane has also sparked a debate in the field. Whichever end of this peptide is exposed to the extracellular environment has a direct impact on its function and how the SSP can interact with the GP2 subunit.
Upon analyzing our structure, we saw that the SSP makes critical interactions with GP2, which help to maintain the native form of the spike complex. Additionally, we found the SSP present in the viral membrane with an unexpected orientation. A topological rearrangement occurs during the formation of the native spike – essentially, one portion of the SSP flips from one side of the membrane to the other. The structure has thus revealed that the SSP plays a critical role in stabilizing the native spike complex.
Upon further inspection of our model, we noticed something unusual. At the membrane-distal part of the spike, there was a blob of density unaccounted for, which seemed to be coiled around the three subunits of the spike. Further analysis of this density indicated that it belonged to matriglycan – the putative receptor of our virus (see Figure 2). At first, this finding didn’t make any sense – how could matriglycan be present in our structure without α-DG? Our sample was purified away from the receptor, and we didn’t see any additional protein attached to Lassa’s spike from our cryo-EM images. All current literature also points to matriglycan only being found on α-DG and not existing in a soluble form.
But then the idea arose: maybe this matriglycan fragment is simply a “placeholder” for the virus to ensure that it successfully escapes from its host cell. During budding, as viral particles are freed into the extracellular environment, this piece of matriglycan would function to actively prevent the spike from interacting with α-DG present on the dying cell. If the spike of a new virus was to bind erroneously with α-DG on this “sinking ship”, it would be a death sentence. But once the virus is far away from the dying cell – this matriglycan fragment would dissociate, and the spike would be ready to bind any new α-DG which it encounters. To validate this hypothesis, we wanted to see if an excess of matriglycan fragments would prevent Lassa from binding α-DG. Indeed, this was the case - saturating the environment with synthetically produced matriglycan dramatically decreases the ability for Lassa to infect, as its spike is unable to bind available α-DG.
 Figure 2: The receptor binding site of the Lassa spike present with its matriglycan fragment (shown in green & orange for glucuronic acid & xylose subunits, respectively).
Figure 2: The receptor binding site of the Lassa spike present with its matriglycan fragment (shown in green & orange for glucuronic acid & xylose subunits, respectively).
But where does this “placeholder” come from? And what are the kinetics for its dissociation? We are just beginning to understand the role of this matriglycan fragment, but the picture is becoming clearer. Lassa is a virus that uses an assortment of techniques to maximize the likelihood that each virion succeeds with infection. “Old World” arenaviruses (those endemic to the African continent) like Lassa have evolved to use an unusual strategy to successfully escape from their dying host and to migrate to an uninfected cell. Although numerous questions still remain – determining the role of this “pre-loaded” receptor, alongside an elusive yet structurally important signal peptide, may help pave the way for novel therapeutics to combat this lethal pathogen.
The manuscript can be accessed at: https://rdcu.be/cG9cw
References
- McAuley, J. L., Gilbertson, B. P., Trifkovic, S., Brown, L. E. & McKimm-Breschkin, J. L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 10, 39 (2019).
- J., J. R. J., Alexander, T., W., F. H. & A., G. B. Bridging the Gap: Virus Long-Distance Spread via Tunneling Nanotubes. J. Virol. 94, e02120-19 (2022).
- Lo Iacono, G. et al. Using modelling to disentangle the relative contributions of zoonotic and anthroponotic transmission: the case of lassa fever. PLoS Negl. Trop. Dis. 9, e3398 (2015).
- Nunberg, J. H. & York, J. The curious case of arenavirus entry, and its inhibition. Viruses 4, 83–101 (2012).
- Froeschke, M., Basler, M., Groettrup, M. & Dobberstein, B. Long-lived signal peptide of lymphocytic choriomeningitis virus glycoprotein pGP-C. J. Biol. Chem. 278, 41914–41920 (2003).
- Yoshida-Moriguchi, T. & Campbell, K. P. Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology 25, 702–713 (2015).
- Hara, Y. et al. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl. Acad. Sci. 108, 17426–17431 (2011).
- Inamori, K. et al. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science 335, 93–96 (2012).
- Hastie, K. M. et al. Structural basis for antibody-mediated neutralization of Lassa virus. Science 356, 923–928 (2017).
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in