A ReCIPE to Rescue Immunotherapy in Cancer
Published in Cancer

Immune checkpoint inhibitor (ICI) immunotherapy works in a minority of cancers. Unfortunately, even in cancers where ICI therapy works it is common for cancers to develop treatment resistance. In some cancers like metastatic melanoma, there can be few alternative therapies after ICI treatments have failed; in many cases, this treatment resistance leads to death.
What drives ICI resistance in melanomas and other cancers? Many cancers subvert the immune system by shedding immunosuppressive molecules into the blood. For example, PD-1 is the target of nivolumab and pembrolizumab, which are the most commonly used ICI therapeutics in melanoma. These antibodies block PD-1 on the surface of immune cells from being engaged by PD-L1 on the surface of cancer cells. By interrupting this “don’t eat me” signal, anti-PD-1 ICI therapeutics free the immune system to go after cancer cells. We and others previously showed that cancers can secrete soluble PD-L1 (sPD-L1) or exosomal PD-L1 (exoPD-L1) from their surfaces. When sPD-L1 is secreted at high concentrations in the blood, it overwhelms PD-1 on immune cells and outcompetes anti-PD-1 ICI to block anti-tumor immunity. That causes resistance to ICI therapies. This same process can happen with anti-PD-L1 and other ICI, as cancers also pepper the immune system with soluble forms of TIM3, LAG3, and PD-L2, among others. In all, these soluble immune decoys (“SIDs”) drive resistance and death.
What can we do about SIDs in melanoma and other cancers? Our group has experience with a medical procedure that has been around since 1970 called “therapeutic plasma exchange” (TPE). In TPE, we draw blood out of a vein, remove its fluid (plasma) component, and return its cellular contents (red blood cells, white blood cells, and platelets) along with replacement fluid (in this case, purified albumin with appropriate electrolytes and a mild anticoagulant) into another vein. The result is the removal of soluble substances from the blood. While it may sound barbaric, the procedure usually causes minimal discomfort. We previously showed that TPE removes soluble PD-L1 from the blood. In another study, we found that limited radiation to some melanomas can induce the kinds of immune cells that respond to ICI treatment. This led to the ReCIPE-M1 trial (Rescuing Cancer Immunotherapy with Plasma Exchange in Melanoma), where we used minimal radiation, TPE, and ICI re-challenge to treat ICI-refractory melanoma.
In our study, we treated 18 people who had (1) melanoma that was resistant to ICI therapy and (2) high sPD-L1 concentrations in the blood. Each person underwent radiation treatment to a small minority of visible cancer sites followed by three days of TPE followed by ICI re-challenge.
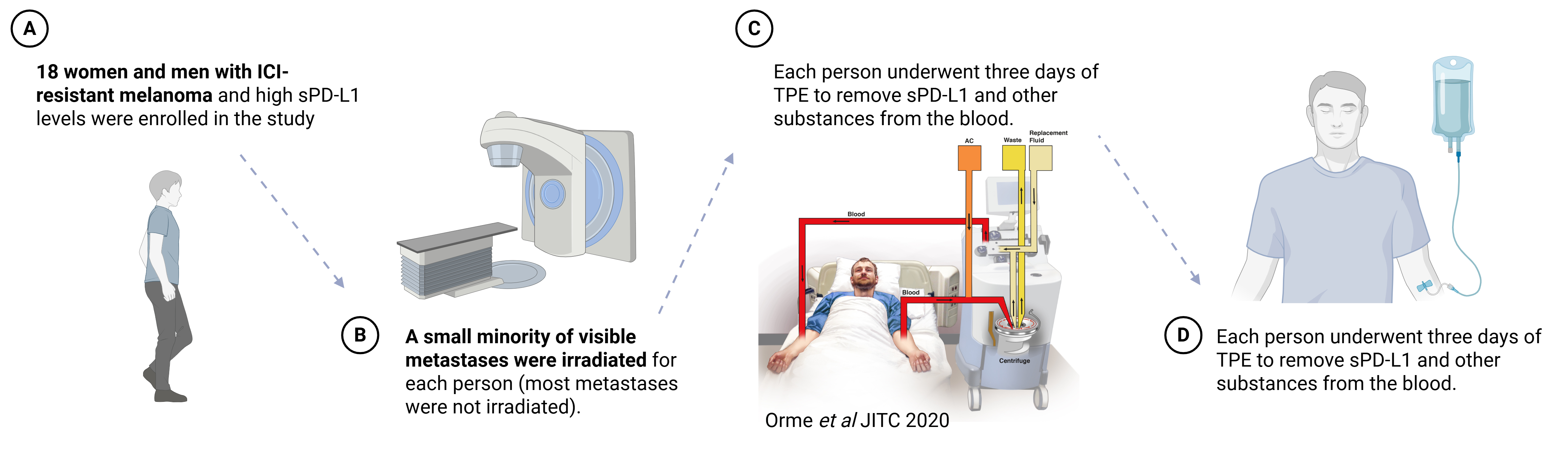
[Created in BioRender. Orme, J. (2025) https://BioRender.com/raf1qvu]
The first question we were trying to answer in this study is whether this treatment approach (specifically, TPE to remove sPD-L1 from the blood) is feasible in men and women with melanoma. While we learned a lot along the way, we found this approach was both safe and effective at removing sPD-L1 as we had hoped.
The second question we were trying to answer in this study is whether this could work to overcome resistance. Our main measure of success was the overall response rate—meaning the percent of people on the study who saw a significant reduction in the size of visible non-irradiated tumors after our treatment—in comparison to a historical control group. In this study, the overall response rate was 61%, which appeared much better than comparable historical controls. We also found that the people in the study who had a long-lasting suppression of their sPD-L1 levels after TPE enjoyed much better clinical outcomes than their peers.
These findings led us to dig deeper, trying to establish how radiation and TPE actually worked to make ICI re-challenge successful against the cancer. We measured different types of immune cells in the blood and found that changes in those immune cell populations predicted how well the treatment was working. This suggests that the therapy works for the reasons we would expect it to work: in short, better activation of the immune system by the immunotherapy was possible and effective after TPE and ICI re-challenge.
We also know that sPD-L1 isn’t the only SID in the blood of men and women with ICI-refractory melanoma. We measured more than 5,000 substances in the blood, including important known SIDs, and found that these were also reduced by TPE. Interestingly, most of these bounced back within a few weeks of TPE.
These are very promising results, but there are some caveats to consider. First, there was only one group of people here, and they all got the same treatment. There wasn’t a placebo group to compare in the trial, so we relied on historical controls. While our results look very promising compared to historical controls (for example, 9% response rates in ICI monotherapy switch in a comparable study), we did not randomly assign anyone in this study to a different treatment. As a result, we can’t know for sure whether these people did well just because of our treatment or due to chance. That will require a larger study, which we have planned. Second, it’s not clear which part of the therapy—limited radiation or TPE—had the biggest effect on making ICI re-challenge work for many of the people in our study because everyone received both radiation and TPE. Although we did find that people who had the strongest and longest reduction in sPD-L1 experienced much better results, we need randomized studies to disaggregate how much each of these interventions contributes.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in