A reporting guideline for systematic reviews of outcome measurement instruments (OMIs): PRISMA-COSMIN for OMIs 2024
Published in Healthcare & Nursing, Biomedical Research, and Public Health
Outcome measurement instruments (OMIs) play a critical role in assessing health outcomes both in clinical practice and in research. Different types of OMIs exist, such as questionnaires or patient-reported outcome measures (PROMs), clinical rating scales or performance-based tests. While there are many OMIs that measure the same outcome domain, selecting the most suitable OMI requires a comprehensive evaluation of the measurement properties, feasibility and interpretability aspects. Such evaluations can be achieved through the conduct of systematic reviews.
Despite various methods available for conducting systematic reviews of OMIs, there is a continuing lack of comprehensive reporting of methods and findings in these reviews’ publications. This incomplete reporting limits reproducibility and hinders the selection of the most appropriate OMI for specific applications. Recognizing this gap, we recently developed a reporting guideline specifically tailored for systematic reviews of OMIs, called PRISMA-COSMIN for OMIs 2024.
New reporting guidance
To develop PRISMA-COSMIN for OMIs 2024, we adapted the PRISMA 2020 framework by adding new items, modifying existing ones, and ensuring the relevance of all items to systematic reviews of OMIs. This process was an international collaborative effort, and involved a Delphi study, workgroup meetings, and extensive pilot testing. We engaged important partners, including patients and members of the public throughout the development process. As patient and public involvement (PPI) in the development of reporting guidelines is relatively new, we shared our lessons learned in the form of 17 recommendations that future reporting guideline developers can refer to (Figure) in a commentary recently published in Research Involvement and Engagement. Key to the successful integration of patient and public involvement were:
- Limiting power imbalances by having a patient on the research team.
- Organizing a preparatory meeting for patients and public members to go over key project details and concepts.
- Remaining flexible in how patients and public members could contribute.
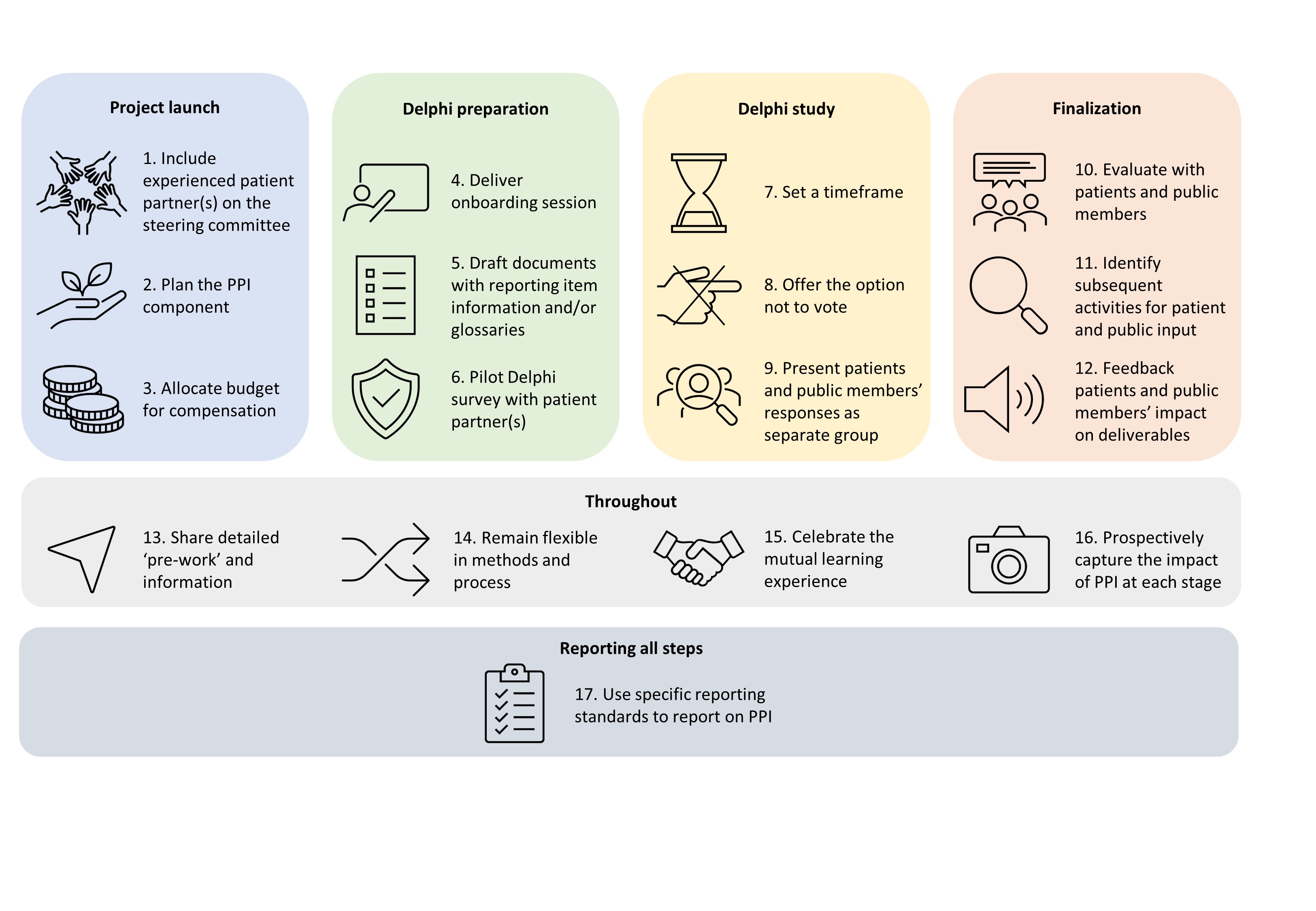
Figure: 17 steps to meaningful Patient and Public Involvement (PPI) in reporting guideline development
The impact of PPI on the guideline was evident, as reporting items that may otherwise have been disregarded were included, such as the feasibility and interpretability of OMIs, recommendations on which OMI (not) to use, and the plain language summary. Moreover, suggestions of patients and members of the public for rewording some of the items made the guideline much clearer.
The resulting PRISMA-COSMIN for OMIs 2024 guideline comprises of a checklist for full systematic review reports and one for abstracts, along with Explanation and Elaboration (E&E) documents. The checklist for full reports contains 54 (sub)items covering various sections of a systematic review report, from the article’s title to the discussion (Table).
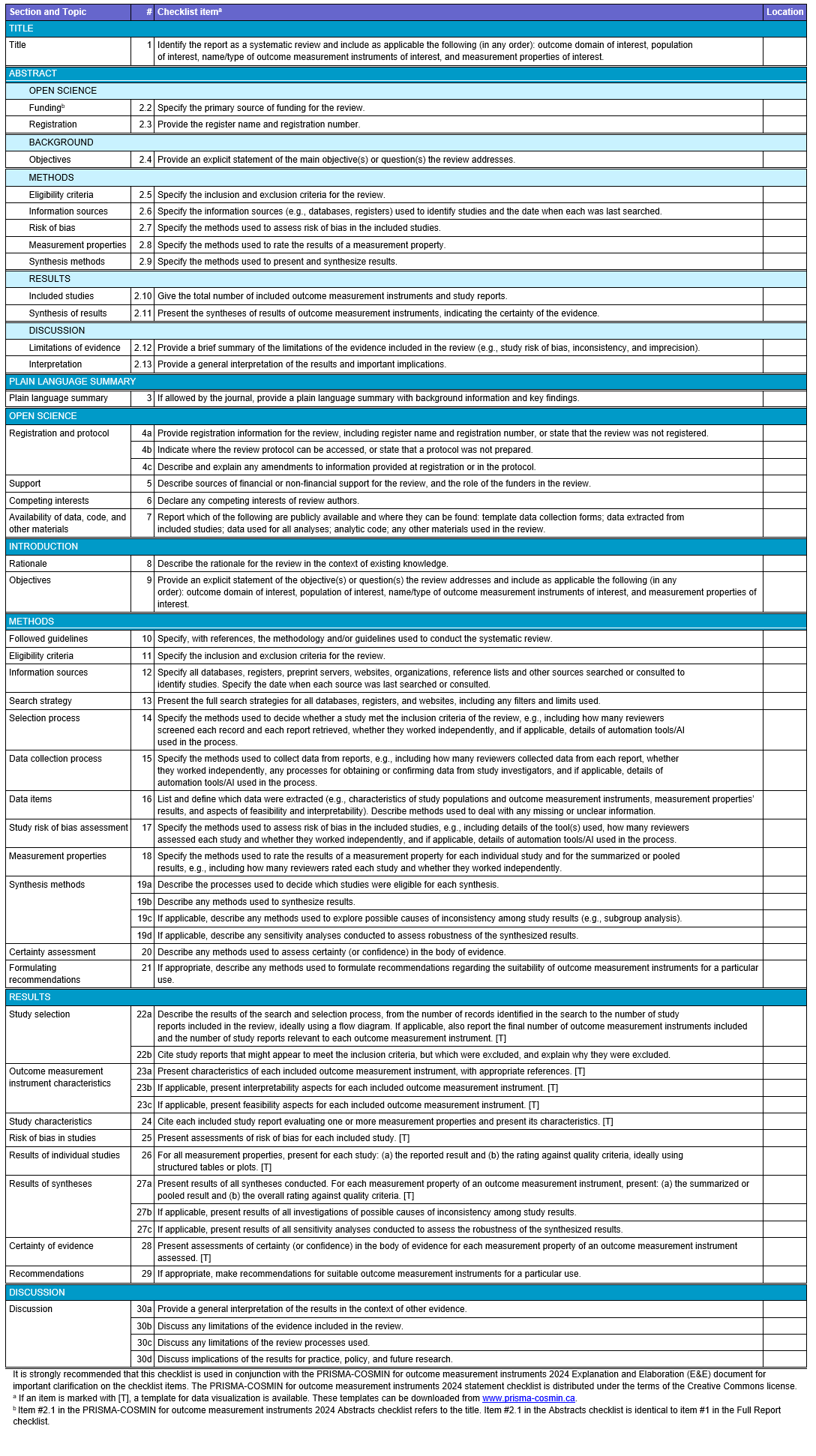
Tools for authors and reviewers
PRISMA-COSMIN for OMIs 2024 can be used to guide the reporting of all systematic reviews of OMIs, where at least one measurement property of at least one OMI is evaluated, irrespective of the methodology used to conduct the review. The manuscript detailing the development of PRISMA-COSMIN for OMIs was co-published open access in July 2024 in Health and Quality of Life Outcomes, Journal of Clinical Epidemiology, Journal of Patient-reported Outcomes, and Quality of Life Research. This joint publication underscores the significance of PRISMA-COSMIN for OMIs 2024 to the research community.
Looking ahead, we anticipate that PRISMA-COSMIN for OMIs 2024 will contribute to enhanced quality and transparency of reporting of systematic reviews of OMIs. It can be used by authors who are drafting their systematic review report, as well as journal editors and peer reviewers to appraise the completeness of reporting. To encourage widespread adoption and implementation, we put in place a comprehensive knowledge translation strategy, including making the guideline available on the websites of the EQUATOR network, PRISMA, and COSMIN. A website dedicated to PRISMA-COSMIN for OMIs 2024 was also created: www.prisma-cosmin.ca. All resources, including the downloadable fillable checklists (in PDF and Word) are available on this website. In addition, we launched an ongoing social media campaign, produced a short explanatory video, one-page tip sheets for each checklist item, and developed patient-focused materials, which all can be found on the website. As of July 2024, authors who register their OMI systematic review in PROSPERO are sent an email alerting them to PRISMA-COSMIN for OMIs 2024. We will evaluate and monitor the implementation of the guideline, and welcome feedback on its use.
Follow the Topic
-
Quality of Life Research

This is an international, multidisciplinary journal of original research, theoretical articles, and methodological reports related to the field of health related quality of life (HRQL), in all the health sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Quality of life in people with mental disorders – beyond global scores
The World Health Organization's 65th World Health Assembly stated that the global burden of mental disorders accounts for 25–33% of all disabilities and highlighted the substantial impact on family members and carers. The WHO's "Comprehensive mental health action plan 2013–2030" stresses the lack of adequate health service provision in this area. Research into evaluating the efficacy and effectiveness of interventions and mental health services often focus on measures of symptom severity as key outcomes. But policy agendas as well as patients’ views suggest considering impacts beyond pure symptom load including a broad range of experience and a consideration of a dimension of "recovery". The assessment of health-related quality of life and quality of life ((HR)QoL) and interventions targeting these constructs have been articulated as a way to improve treatment outcomes during mental health service provision.
The purpose of this article collection is to increase the awareness of and reliance on the multidimensional nature of (HR)QoL by moving beyond global (HR)QoL scores, both in research as well as applied (clinical) settings.
For the purposes of this call, we conceptualize people living with "mental disorders" broadly, as for example in line with diagnoses (DSM, ICD), transdiagnostic approaches, or being in treatment that focuses on their remediation.
In this article collection we encourage submissions of research and clinical practice using nuanced approaches to (HR)QoL in people living with mental disorders. We envisage that such papers will increase our understanding of (HR)QoL in diverse settings and samples, which may also prompt the reshaping of interventions and services to target specific (HR)QoL deficits or increase capabilities.
To contribute to this nuanced discussion, submissions should firstly position their research clearly as addressing QOL or HRQL. While authors are invited to position their research in other relevant frameworks, the editors suggest as a reference point the WHO (1994) definition of QoL as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns”; and the HRQL focus on the impacts of health conditions, their consequences, and treatments on multiple aspects of people’s quality of life, such as for example level of ability, daily functioning, and ability to experience a fulfilling life.
Secondly, many (HR)QoL instruments have been developed as multidimensional tools, offering insights into several related domains at a time. A brief, non-exhaustive list of examples of instruments measuring multiple (HR)QoL dimensions are the WHOQoL (psychological health, physical health, environment, social relationships); the SF-36 (physical functioning, physical role limitations, bodily pain, general health perceptions, energy/vitality, social functioning, emotional role limitations); the MANSA (Life and Health, Living Environment); the MSQoL (physical health, vitality, psychosocial, affective, material satisfaction, spare time). Any paper submitted to the call should use appropriate methods to test claims about the multidimensional nature of their approach, at least as supporting or sensitivity analyses.
Eligible manuscripts may focus on:
- investigating the potential causal impact of interventions or service provision models on particular dimensions of (HR)QoL; such submissions may use confirmatory or exploratory approaches and use data from controlled or naturalistic settings;
- the understanding of the nature, appropriateness, or other aspects of the meaning and use of these dimensions in, or ideally with, the target population, using in-depth explorations through qualitative inquiries;
- examining different factors (i.e., sociodemographic, psychosocial, clinical) that may act as longitudinal determinants of, or display associations with, (HR)QoL dimensions in people with mental disorders; especially encouraged are explorations relating to social determinants, inequities and inequalities that matter in the particular research and service contexts;
- performing investigations of the viability of domain scores with contemporary psychometric methods (e.g., via confirmatory bifactor analyses or other models that allow to gauge the differential contribution of domains of an instrument);
- explaining the use of domain information in practice contexts by focusing the interventions only on specific domains where patients report major difficulties (i.e. social relationships; employment);
- performing investigations and develop methodological proposals on how to research relationships between measures of the severity of mental disorder (symptoms) and (HR)QoL, with often overlapping item content in measures of both constructs (DOI: 10.1007/s40273-020-00972-w);
- comparing QoL dimensions across people with different diagnoses of mental illness (i.e., schizophrenia-spectrum, mood, anxiety disorders, etc.);
- relating QoL dimensions to dimensional measures of mental illness (e.g., HiTOP or network approaches;
- comparing QoL dimensions across people with mental disorders that may attend different service settings, or may be at different time points of their condition course (i.e. first-episode, chronic patients; inpatients/outpatients, etc.);
- presenting the findings of systematic reviews/meta-analyses of the available evidence regarding the QoL dimension levels, and/or associated factors in people with mental disorders.
Manuscripts may use original data or provide evidence based on secondary analyses. Submissions should employ reporting guidelines relevant to the type of study and applied methodology to transparently and completely report their research. The standard word limit of 4,000 words applies, but it is common for submissions to Quality of Life Research to contain substantial electronic supplements or link out to repository resources to provide all necessary information to understand and engage with the reported research. The use of (pre-)registrations and open science techniques is strongly encouraged to increase transparency and the potential for re-use.
Participation in the article collection:
Submissions of full papers in line with QLR's submission guidelines will be possible from 01.11.2024 in our online submission portal. There is currently no definite end date and we envisage the collection to be open for at least a year.
A submission of a letter of intent is not necessary. If helpful for authors, they are invited to submit a letter of intent for pre-evaluation during this period. A letter should contain a draft title, contact information and institution for all co-authors, and a structured abstract (500 word maximum) that provides detailed information about the manuscript to support the evaluation. Please email the letter to Dr. Brittany Lapin (lapinb@ccf.org) and Dr. Eleni Petkari (epetkari@uma.es) These letters will be handled on an ongoing basis and feedback (invitation to submit the full paper vs. rejection) will be provided within two weeks.
Papers will be published online as part of the collection if accepted after a single-blind peer-review process. Published articles will be allocated to issues on an ongoing basis as soon as possible thereafter.
Publishing Model: Hybrid
Deadline: Jun 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in