A Simpler Approach for Constructing Sophisticated Bicyclic Lactones
Published in Chemistry

We at Prof. Debabrata Maiti's laboratory have been exploring the reactivity of the carboxylate group in activating sp3 C-H bonds for several years now. While we were exploring the C-H functionalization possibilities in the realm of cyclic aliphatic acids, we found this amazing reaction that shows reverse site-selectivity and forms bicyclic lactones with unsaturation in the cyclic ring. We are gonna talk about the story of this project and the journey in between.
How/Why we discovered this project
We all are aware of the beauty of lactones. Its ubiquitous influence can be observed everywhere, from fragrance molecules to pharmaceutically relevant compounds.1,2
But how do chemists prepare lactones?
The most classic textbook reaction that comes to mind is intramolecular esterification. This involves the cyclization of a hydroxy acid or hydroxy ester compound with a leaving group, resulting in the formation of a lactone. Then there are other reactions like Baeyer-Villiger Oxidation and iodolactonization to generate lactones.3
Even though there exists a number of chemical reactions to form lactones, the route is often not straightforward. Either the starting materials are highly designed (not readily available) or there are multiple steps to form the product. All of these count for the reduced atom and step economy of reactions.
With our exploration of carboxylate-assisted sp3 C-H activation, we realized a great opportunity to form not just simple lactones but bicyclic lactones. The prominent challenge was to form lactones in a single step from alkyl carboxylic acid without having to employ any external coupling partner.
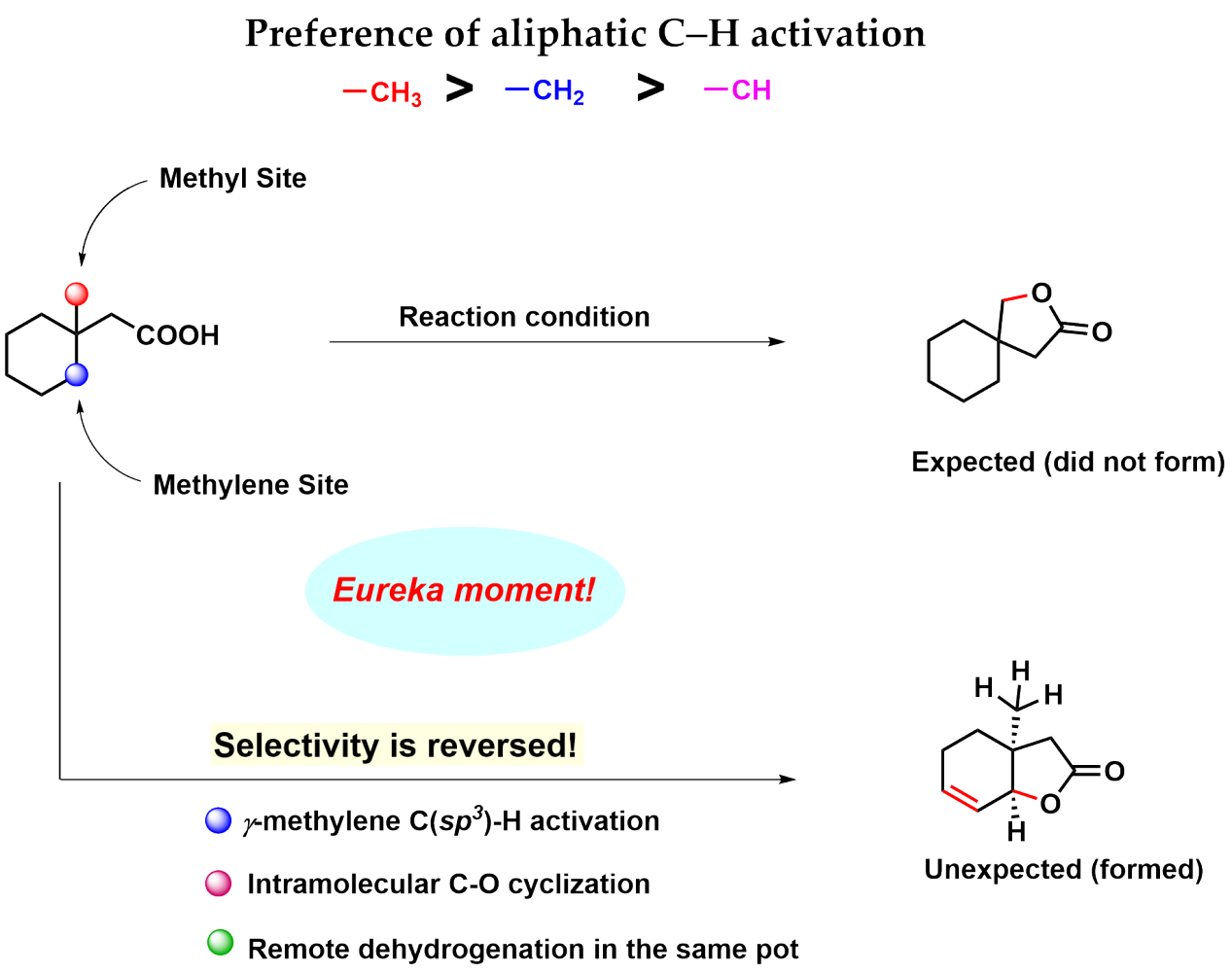
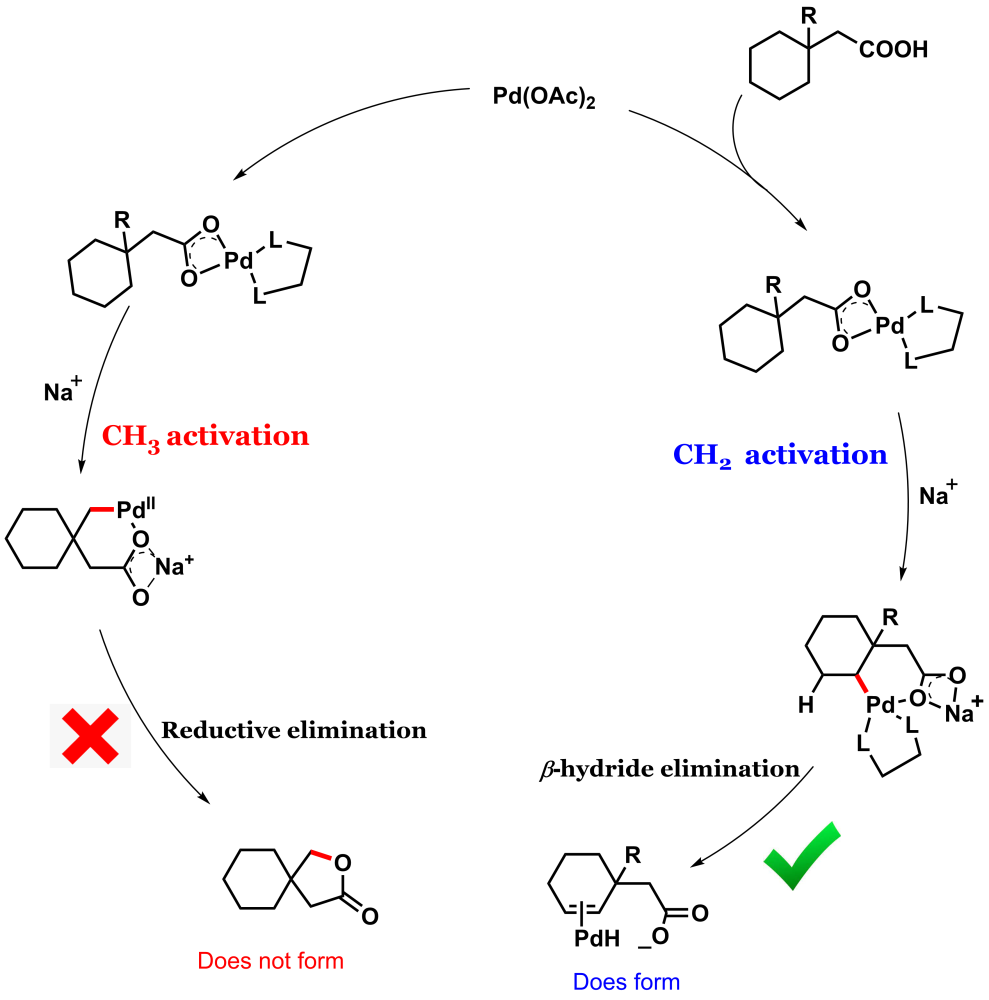
Intramolecular lactonization of the aliphatic carboxylic acid is a disfavored process as evident from the previous literature.4 We came up with a cyclic aliphatic acid 3-methyl cyclohexane acetic acid that has equally accessible gamma methyl and gamma methylene groups.
The conventional knowledge would indicate that methyl group activation is easier than methylene. But the catch with this substrate is that the subsequent step reductive elimination following methyl activation is probably difficult (that's exactly what we found later in our DFT studies) and so it should not proceed.
Whereas methylene activation, though more strenuous but subsequent organometallic step beta-hydride elimination should be more favorable (Figure 1). With this hypothesis, we subjected the substrate to an amenable reaction condition, and voila! we found our thought process to be true.
Utility of the discovered reaction
Some crucial benchmarks for testing the effectiveness of a new chemical reaction are its generality, applications, etc.
Is the reaction equally potent for a diverse class of substrates? Can the reaction be applied for accessing important complex molecules?
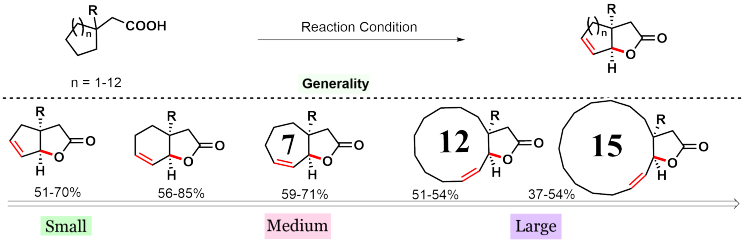
In order to find answers, we assessed a number of different aliphatic acids which contain 5, 6, 7, 12, and 15-membered rings. The protocol was found to be effective for all such substrates, albeit, in different yields. The interesting case is with macro ring containing acids (12 and 15 membered). Macro lactonization has been traditionally considered a challenging reaction as it often required highly diluted conditions and slow addition procedures to prevent oligomerization.5 Nonetheless, we could achieve macro lactonization utilizing the same exact conditions as other classes of substrates.
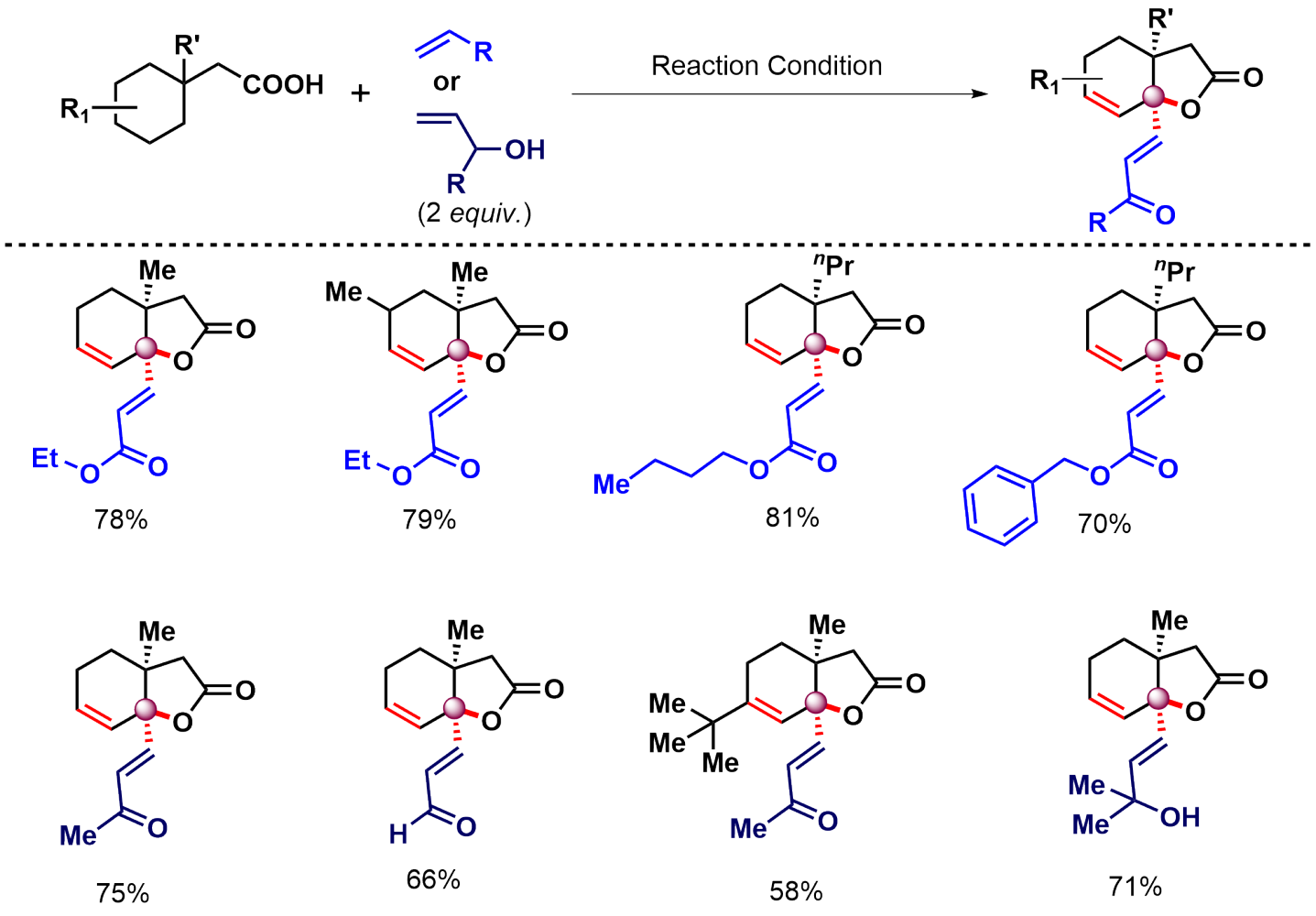
What intrigued us more is when we tried to make the reaction work in the presence of an external partner. We call it the intermolecular version of the reaction. If you don't add anything extra (except the substrate) in the reaction, it forms unsaturated lactones (Figure 2), however, if you add an olefin or an allyl alcohol, it forms unsaturated lactones containing olefins (Figure 3).
The intriguing part is how many things are going on in a single-pot reaction. In the presence of Olefin, here are the events-
- gamma-methylene C-H activation
- C-O cyclization
- Unsaturation in the ring
- gamma-olefination
One of the long-standing goals of synthetic chemists is to make more complex compounds from simpler starting materials (ideally in a single step). This reaction shows the potential of such approaches. Undoubtedly, we are going to witness more such reactions in the coming days where complex molecules could be synthesized from one or two-step reactions (e.g., multicomponent reactions).
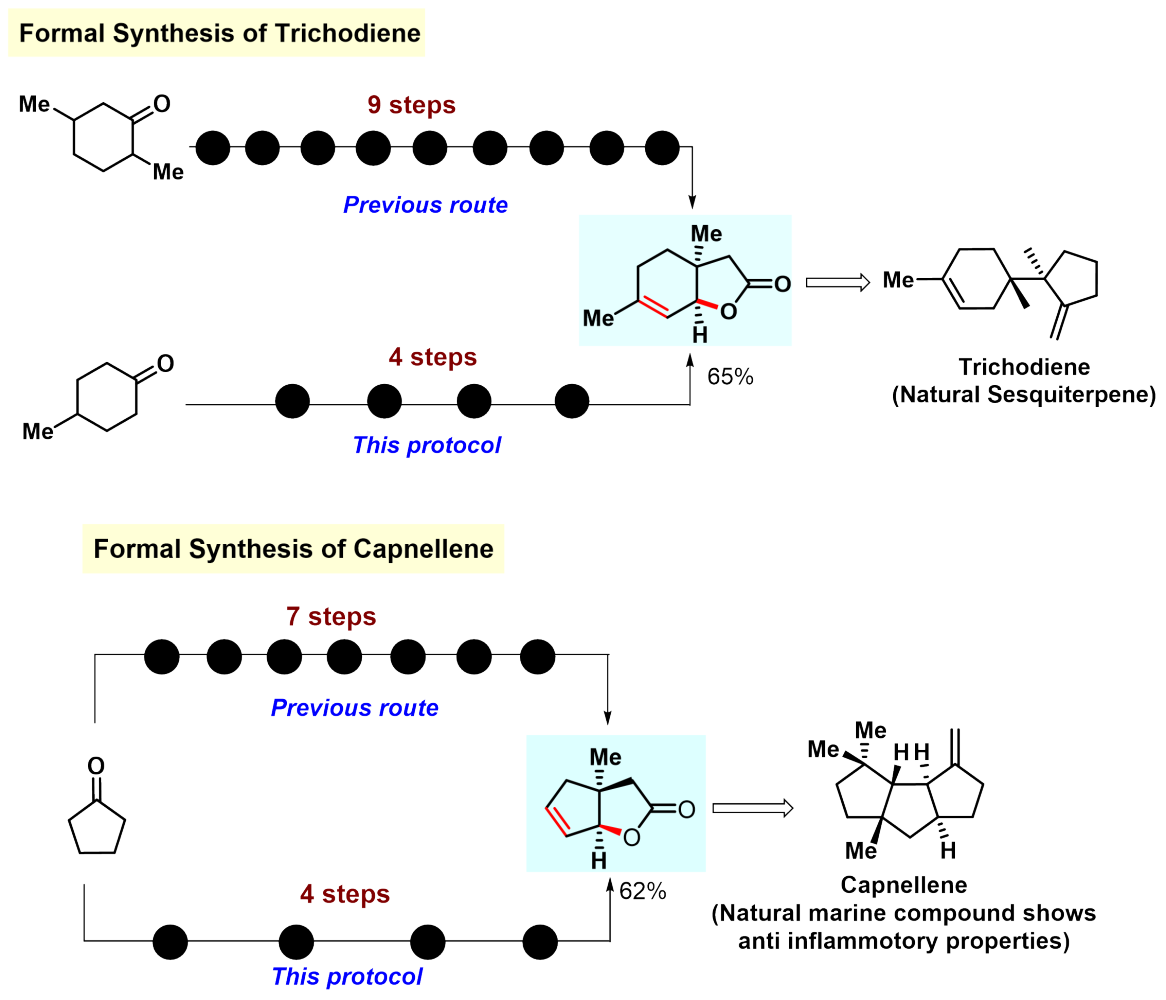
We portrayed a number of instances where the developed protocol could be harnessed to form various natural products or bioactive molecules in fewer steps than before (Figure 4).
The mystery of reverse site selectivity
Reversing the conventional site selectivity is one of the major highlights of this reaction. This was always in our minds and so we wanted to find a rationale for this.
We collaborated with Dr. Xinglong Zhang from IHPC, A*STAR, Singapore to unravel the mystery. We found the substrate to be in a unique position to command reverse site selectivity. Although the methyl activation is more facile in this case, the subsequent organometallic step (after methyl activation) reductive elimination is unfavorable in this case.
Whereas the methylene activation (though a little more strenuous than methyl activation) is accompanied by a more favorable process of beta-hydride elimination and thus the system favors the methylene activation over methyl (Figure 5). More details are in the paper. It is as if the system exhibits delayed gratification instead of instant gratification.
Conclusion
This reaction is a great example of the capability of weak coordination in activating inert C-H bonds and thereby starting a cascade reaction. Sometimes a gentle push is more effective than doing it the hard way. While strong coordination is considered better for forming organometallic complexes, weak coordination in a reaction can result in fruitful end products.
What about aliphatic ketones, alcohols, esters, etc. functional groups? Can we harness the weak coordination of such functional groups for driving reactions?
We anticipate more exploration in such domains in the coming years, potentially leading to more exciting chemical reactions.
Reference:
1. Kreuger, M. R. O., Grootjans, S., Biavatti, M. W., Vandenabeele, P. & D’Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment Anti-Cancer Drugs 23, 883-896 (2012).
2. Li, G., Kusari, S. & Spiteller, M. Natural products containing ‘decalin’ motif in microorganisms. Nat. Prod. Rep. 31, 1175-1201 (2014).
4. Kao, L.-C.; Sen, A. Platinum (II) catalyzed selective remote oxidation of unactivated C−H bonds in aliphatic carboxylic acids. J. Chem. Soc. Chem. Commun. 18, 1242 (1991)
5. Force, G., Perfetto, A., Mayer, R. J., Ciofini, I., Leboeuf, D. MacrolactonizationReactionsDriven by aPentafluorobenzoylGroup. Angew.Chem.Int. Ed., 60, 19843–19851 (2021)
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in