Society’s essential transition away from fossil-fuels demands new catalysts to facilitate the multi-electron transformations that will underpin new Power-to-X technologies. Current state-of-the-art catalysts are dominated by precious noble metals such as palladium and platinum, and tremendous efforts have been undertaken to maximize the number of atoms exposed on the surfaces of these catalysts by miniaturizing particle sizes.1 New catalysts cannot be designed, however, without accurate and precise understanding of the geometric and electronic structures of the active sites, and yet surface supported nanoparticle catalysts are famously difficult to prepare and characterize without defects.2
One of the strategies to enforce homogeneity of nanoparticles within new materials, has been to confine them within rigid, well-defined host supports, such as metal-organic frameworks.3 These MOFs are constructed from organic linkers (struts) bearing negatively charged groups which tether positively charged inorganic nodes (structural building units) into ordered, polymeric network structures that emerge from the combination of geometries of the organic linker and inorganic nodes. While nanoparticles embedded within MOF architectures have delivered impressive improvements, such approaches necessarily compromise the pore volume of the MOF hosts, and the nanoparticle guests are not as well stabilized by the framework as, say, the inorganic ions inside the structural nodes.
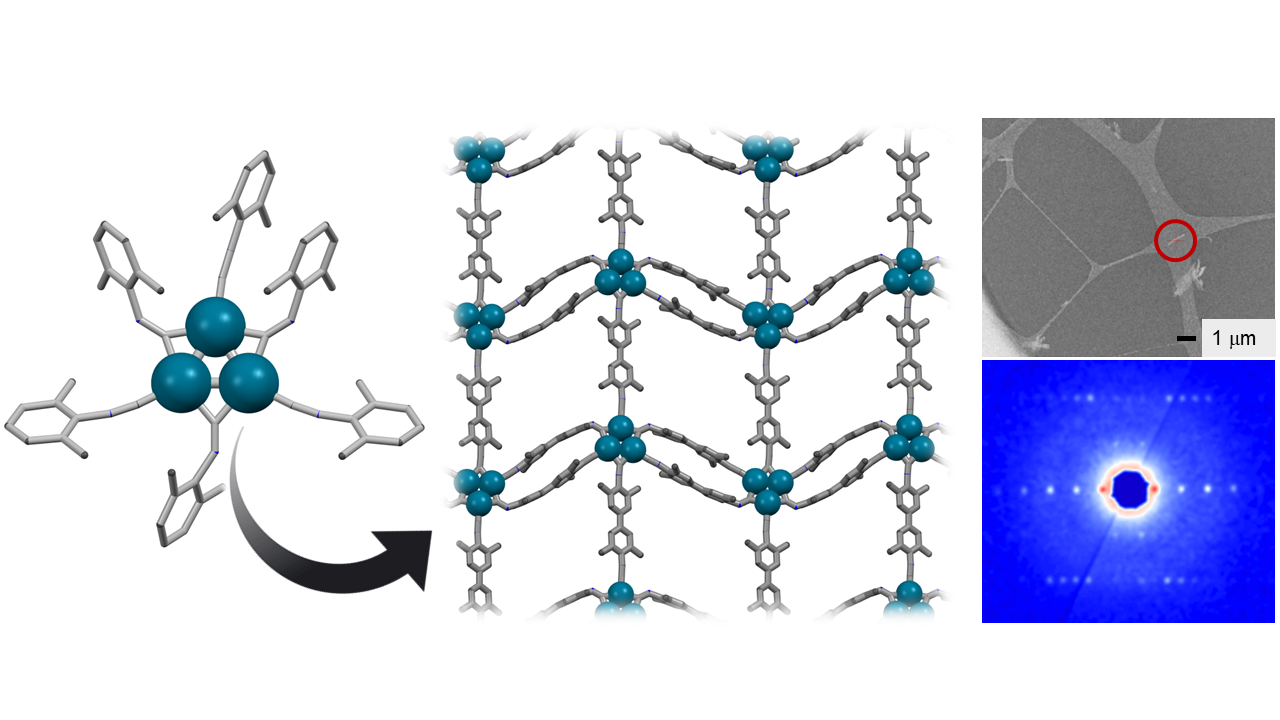
We thought that a more elegant approach would be to construct frameworks using nodes that resemble miniaturized metal nanoparticles—however these clusters are a far cry from the charged ions that typically found in the structural building units of conventional MOFs. Until now, there are only a handful of examples of well-defined frameworks that have been constructed from neutral metal atoms, and all possess only single metal atoms at each structural node.4 This is in a large part due to challenges in growing suitable crystals for single-crystal X-ray diffraction studies, hindering the discovery of such structures. Simply, the electrostatic interactions which hold conventional MOFs together are more reversible than the more covalent interactions which bind charge-neutral (zero-valent) building blocks, which are thus much more likely to assemble statistical, disordered polymers and tiny crystallite grains, unsuitable for structural determination by conventional X-ray crystallographic techniques.
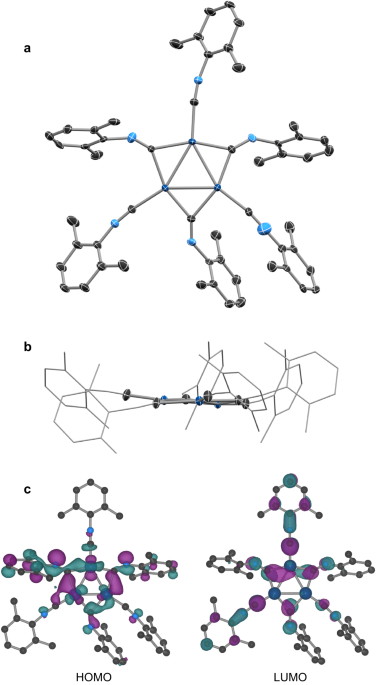
To limit the complexity of our MOF synthesis, we sought to pre-assemble our desired metal-cluster nodes as well-characterized molecular clusters (using well-established coordination chemistry techniques). Our hope was that that we would then be able to tune the rates of ligand exchange reactions by varying the reaction conditions (temperature, concentration, etc.) or synthetic modification of the supporting ligands on the pre-assembled cluster, such that we could realize ordered, crystalline products. We focused on isocyanide (:C≡N−R) donor groups which stabilize electron-rich metal centers (in this case palladium(0)), and the coordination chemistry of related (but less synthetically interesting) carbonyl-supported clusters provides an extremely mature and diverse source of inspiration.4
Preparing a homogenous solution of pre-assembled clusters.
In the presence of two equivalents of an isocyanide (CNXyl in this case), organometallic Pd(0) precursors assemble into triangular Pd3(CNR)6 clusters.5 We started with the xylyl isocyanide (CNXyl, 2,6-dimethylphenylisocyanide), as we wanted to conserve as much as possible the structure of the molecular {Pd0}3 cluster from the precursor in the MOF structural node, and the two methyl groups provide a degree of steric protection and convenient handles for solution state characterization (for example, by nuclear magnetic resonance techniques). While this approach worked in this work, we expect to be able to adapt our strategy to realize other MOFs composed of zero-valent metal clusters. A broad scope of isocyanides, with rich variety of electronic properties and steric profiles are available, which can be matched and tuned to control the rates of network formation as required as we explore the discovery of new frameworks.
Network formation and crystallization.
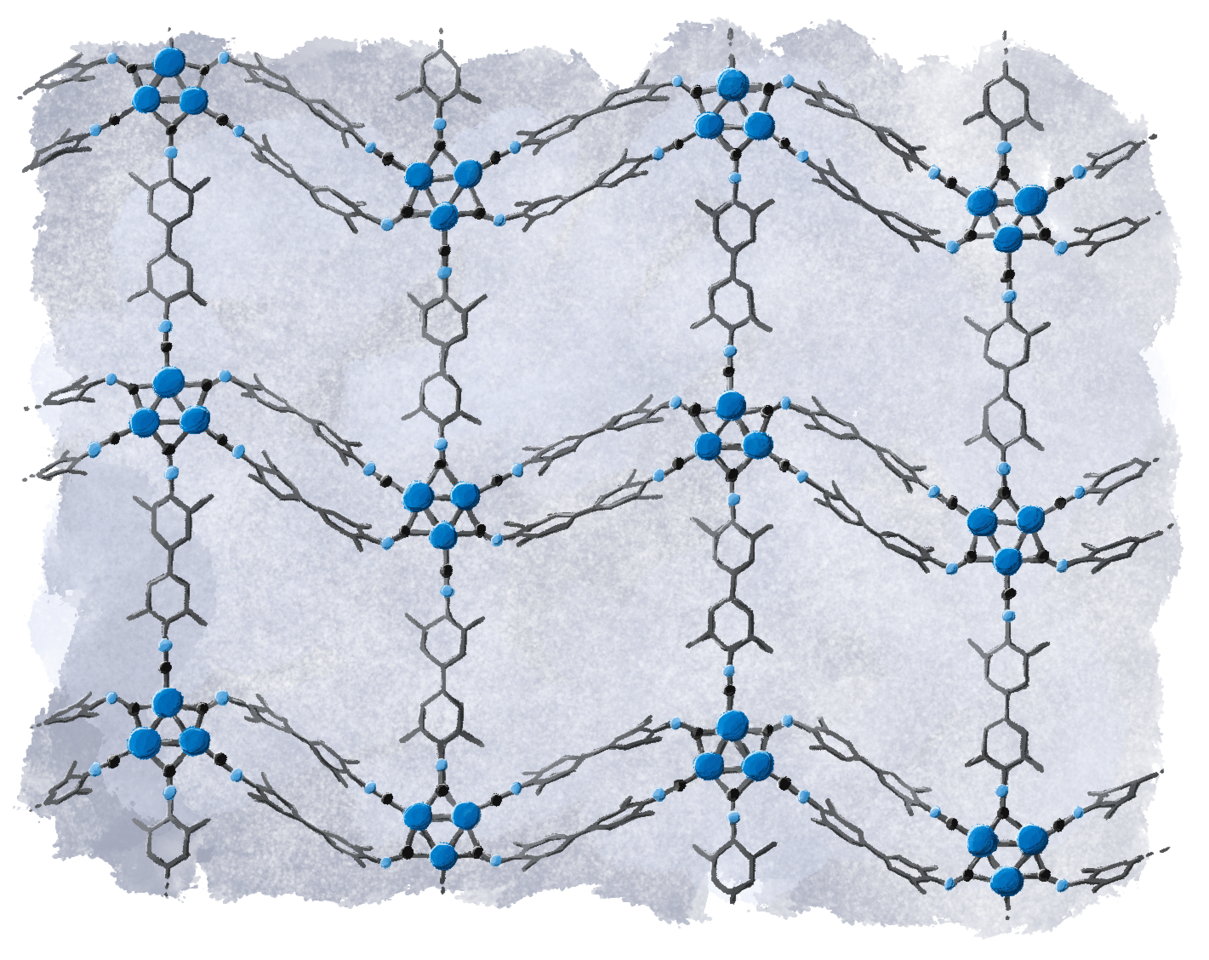
Pd3-MOF precipitated as a red, nanocrystalline powder when a linear organic strut featuring two isocyanide groups was added to a solution of the molecular Pd3 cluster. Complete exchange of the monotopic CNXyl ligand was confirmed by NMR spectroscopy on the liquid phase, and we confirmed that the electronic structure (and zero-valence) of the Pd3 cluster was preserved in the MOF by X-ray absorption spectroscopy and X-ray photoelectron spectroscopy.
Unambiguous structure determination of nanocrystalline Pd3-MOF by electron crystallography.
Crystallography offers unparalleled precision in determining the atomic structure of crystalline materials. However, these rely on obtaining single crystals of sufficient size to collect complete, high-resolution diffraction data. Typically, even at advanced synchrotron X-ray beam lines, crystals need to have dimensions on the order of at least 1 µm. This requirement poses a significant challenge, especially in the discovery of the metal-organic frameworks with zero-valent clusters that we are interested in. Indeed, the crystallites of Pd3-MOF formed during our synthesis were so small, that even the powder X-ray diffractograms displayed only very broad signals with no recognizable (if any) high-resolution features beyond 30° 2θ. Fortunately, recent advancements in continuous rotation 3D electron diffraction (3D-ED or MicroED), and the availability of the first commercially available dedicated electron diffractometers provides promising solutions.6 Electrons interact much more strongly with matter than X-rays, and so by merging 3D-ED data from five individual nanocrystals, we were able to unambiguously determine the atomic structure of the Pd3-MOF.
Properties
Unlike the molecular Pd3 precursor, which decomposes rapidly on exposure to air, Pd3-MOF remains stable when exposed to air and a range of solvents. We also demonstrated that the robust and well-defined {Pd0}3 clusters within Pd3-MOF display catalytic activity that is typical of Pd nanoparticles, specifically the hydrogenation of an unsaturated substrate (styrene).
Conclusion
In conclusion, we are thrilled to present our modular strategy, which we believe can be expanded to the plethora of other clusters known in coordination chemistry. This work also highlights the importance of electron crystallography in the discovery of nanocrystalline materials that are typical of such zero-valent MOF syntheses. We are excited to see how these strategies evolve and contribute to advancements in catalysis, as a means to control and characterize the atomic and electronic structures of the active sites within this new class of material.
References
- (a) Liu L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018); (b) Chen, Y. et al. Isolating single and few atoms for enhanced catalysis. Adv. Mater. 34, 2201796 (2022); (c) Ding, J. et al. Advances in the electrocatalytic hydrogen evolution reaction by metal nanoclusters based materials. Small 18, 2204524 (2022); (d) Zhang, B., Chen, Y., Wang, J., Pan, H. & Sun, W. Supported sub-nanometer clusters for electrocatalysis applications. Adv. Funct. Mater. 32, 2202227 (2022).
- (a) Hayton, T. W., Humphrey, S. M., Cossairt, B. M., Brutchey, R. L. We need to talk about new materials characterization. Inorg. Chem. 62, 13165–13167 (2023); (b) Akbashev, A. R. Electrocatalysis goes nuts. ACS Catal. 12, 4296–4301 (2022); (c) Munarriz, J., Zhang, Z., Sautet, P., Alexandrova, A. N. Graphite-supported Ptn cluster electrocatalysts: Major change of active sites as a function of the applied potential. ACS Catal. 12, 14517–14526 (2022).
- (a) Fortea-Pérez, F. R. et al. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 16, 760–766 (2017); (b) Kollmannsberger, K. L., Kronthaler, L., Jinschek, J. R. & Fischer, R. A. Defined metal atom aggregates precisely incorporated into metal-organic frameworks. Chem. Soc. Rev. 51, 9933–9959 (2022).
- (a) Sikma, R. E., Balto, K. P., Figueroa, J. S., Cohen, S. M. Metal-organic frameworks with low-valent metal nodes. Angew. Chem. Int. Ed. 61, e202206353 (2022); (b) Obeso, J. L. et al. Low-valent metals in metal-organic frameworks via post-synthetic modification. Angew. Chem. Int. Ed. 62, e202309025 (2023); Voigt, L., Larsen, R. W., Kubus, M. & Pedersen, K. S. Zero-valent metals in metal-organic frameworks: fac-M(CO)3(pyrazine)3/2. Chem. Commun. 57, 3861–3864; (d) Andersen, C. E. et al. Vapor-phase synthesis of low-valent metal-organic frameworks from metal carbonyl synthons. J. Mater. Chem. C 11, 11460–11465 (2023); (e) Angew, D. W. et al. Crystalline coordination networks of zero-valent metal centers: formation of a 3-dimensional Ni(0) framework with m-Terphenyl diisocyanides. J. Am. Chem. Soc. 139, 17257–17260 (2017).
- (a) Dyson P. J. Catalysis by low oxidation state transition metal (carbonyl) clysters. Coord. Chem. Rev. 248, 2443–2458 (2004); (b) Cesari, C., Shon, J.-H., Zacchini, S. & Berben, L. A. Metal carbonyl clusters of groups 8–10: synthesis and catalysis. Chem. Soc. Rev. 50, 9503–9539 (2021).
- Christofides, A. Xylyl isocyanide platinum and palladium complexes. J. Organomet. Chem. 259, 355–365 (1983).
- (a) Gruene, T. et al. Rapid structure determination of microcrystalline molecular compounds using electron diffraction. Angew. Chem. Int. Ed. 57, 16313–16317 (2018); (b) Jones, C. G. et al. The CryoEM method MicroED as a powerful tool for small molecule structure determination. ACS Cent. Sci. 4, 1587–1592 (2018); (c) Gruene, T. & Mugnaioli, E. 3D Electron diffraction for chemical analysis: Instrumentation developments and innovative applications. Chem. Rev. 121, 11823–11834 (2021); (d) Truong, K.-N et al. Making the most of 3D electron diffraction: Best practices to handle a new tool. Symmetry 15, 1555 (2023).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in