AAV-CXCL9 Combination Immunotherapy: A Promising Strategy in the Treatment of Glioblastoma
Published in Cancer, Protocols & Methods, and Pharmacy & Pharmacology
Glioblastoma and Immunotherapy
Glioblastoma (GBM) is a type of brain tumor that is hard to treat. In the past, treatments like radiation, chemotherapy, and surgery haven't worked well to cure the disease. However, there's new hope with immunotherapy, a type of treatment that uses drugs to help the body's immune system fight cancer.
Despite some successes with immunotherapy, data from clinical trials show that only a few patients experience significant benefits, such as extensions in life expectancy after treatment. This lack of effectiveness is due to how glioma cells, the malignant cells that form GBM, continue to grow and spread. These cells can avoid detection by the immune system and are resistant to standard treatments.
To address this challenge, combining different immunotherapy drugs becomes crucial. This approach aims to enhance the immune system's ability to find and destroy these tumor cells, making it a leading strategy in treating brain tumors. However, developing these immunotherapies is difficult. Even at low doses, they can be toxic, causing patients to suffer from various side effects. Additionally, the blood-brain barrier limits how well these drugs can reach the tumor, reducing their effectiveness.
Moreover, GBM creates a suppressive environment around the tumor. This means the tumor interacts with our immune cells in a way that makes them protect the tumor instead of attacking it. This further complicates immunotherapy because even if immune cells are directed to kill the tumor, there are other immune cells that protect the tumor, weakening the impact of single agent immunotherapy efforts.
To overcome the challenges posed by GBM, we developed an innovative combination of immunotherapy treatments.
AAV-CXCL9 Recombinant Gene Therapy
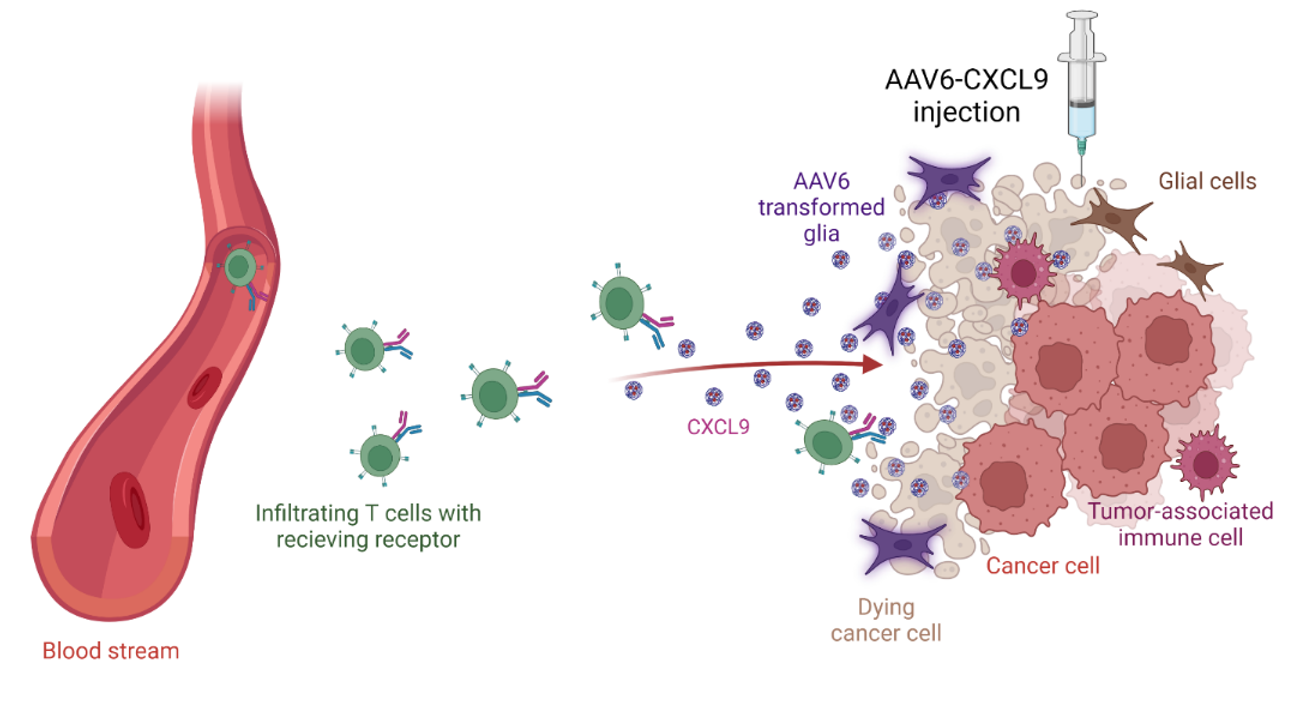
The GBM tumor environment is known for its lack of lymphocytes, which are immune cells capable of killing tumor cells. This is due to the absence of signals needed to activate and guide these cells to the tumor site. To tackle this issue, we've developed an innovative therapy using adeno-associated virus (AAV) to deliver a signaling molecule called CXCL9.
AAV is a type of virus containing single-stranded DNA that can be modified to carry specific genes into target cells. In our approach, we replaced the viral DNA with the CXCL9 gene. Once administered, AAV transports this gene to target cells. Consequently, these cells produce CXCL9 as a protein, which signals lymphocytes to activate and migrate to the tumor. Once there, lymphocytes recruit other immune cells and directly participate in tumor cell killing.
By restoring lymphocyte presence in the tumor, our combination therapy addresses the immune-suppressive environment typically associated with GBM. Moreover, delivering this treatment directly into the brain bypasses the blood-brain barrier, a longstanding obstacle for GBM therapies. AAV therapies are also less toxic since they are not pathogenic which means they cannot replicate in human cells. Overall, AAV's precision in delivering diverse genetic material with minimal risk makes it an ideal vector for this therapy.
Visualizing the Tumor Landscape with AAV
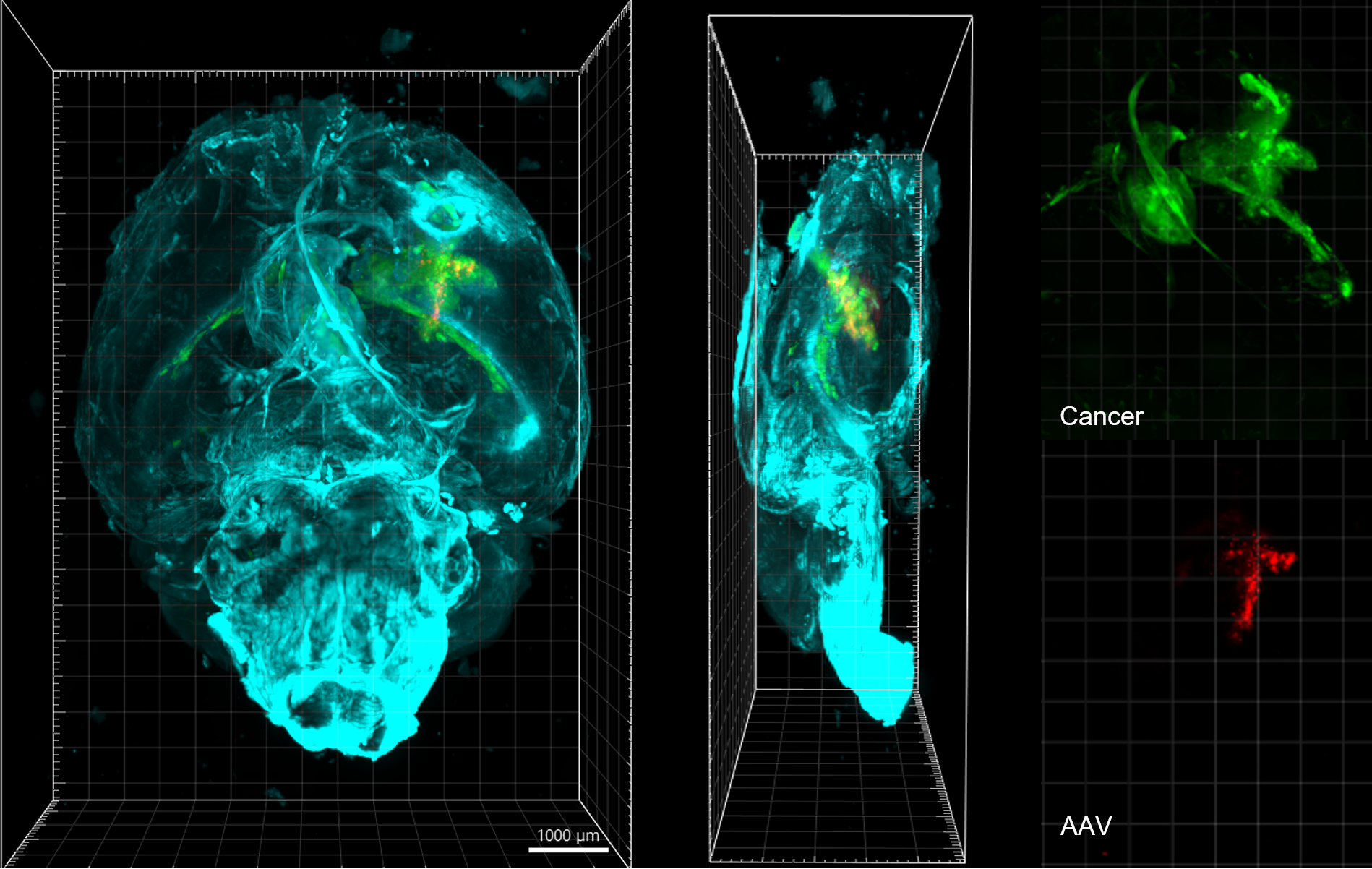
To better understand the cells associated with GBM, we used advanced 3D microscopy. This technique allowed us to see which cells are affected by AAV and how they are positioned relative to the tumor.
When our novel AAV-CXCL9 therapy was being developed, we incorporated a fluorescent gene into the AAV. So, when cells took up CXCL9 DNA, they emitted fluorescence under microscope lasers, enabling us to confirm successful gene transfer.
Microscope imaging verified that our AAV-CXCL9 therapy effectively delivered the CXCL9 gene into astrocytes, cells that are unique to our central nervous system. Unlike tumor cells, astrocytes can continuously manufacture CXCL9 suggesting that our treatment can be delivered as a single dose.
Anti-PD-1 and AAV-CXCL9: A Combined Therapeutic Approach
Historically, single-agent therapies do not improve patient survival in GBM. Therefore, new treatment approaches that combine different types of immunotherapies are predicted to enhance their effectiveness.
Programmed death-1 (PD-1) is a protein found on T cells, a type of immune cell, that normally acts to suppress the immune response and prevents T cells from attacking cancerous cells. PD-1 inhibitors (also known as immune checkpoint inhibitors), a common form of immunotherapy, reverses this suppression and enables T cells to attack cancer cells. Although effective in treating other forms of cancer, PD-1 inhibitors have failed to treat GBM, as seen in clinical trials.
Recognizing the potential of anti-PD-1 to reprogram T cells, we hypothesized that combining it with AAV-CXCL9 therapy could enhance treatment outcomes. Our research showed that when AAV-CXCL9 is combined with anti-PD-1, it improves the ability of lymphocytes to kill tumor cells after they are recruited to the tumor site through CXCL9 signaling. Therefore, anti-PD-1 complements the therapeutic actions of AAV-CXCL9 therapy, highlighting the benefits of combining these approaches.
Results and Future Directions
To assess the efficacy of our treatment, we studied the immune cell composition in brain tissue before and after treatment. We hypothesized that cells transduced with CXCL9 would release signals that attract lymphocytes to the tumor site, promoting an immune response. To investigate this, we used a technique called single cell RNA sequencing (ScRNAseq), which allows us to analyze different cell types within a tissue. We applied ScRNAseq to brains from both treated and untreated groups to track which cells, specifically immune cells, migrated to the brain due to our therapy.
Our ScRNAseq analysis revealed a notable increase in the infiltration of immune cells, including lymphocytes, to the tumor site in groups treated with AAV-CXCL9 therapy. There, immune cells mounted an inflammatory response aimed at attacking the tumor. This finding supports the extended survival observed in groups receiving combination therapy, highlighting the effectiveness of our approach in enhancing immune response against brain tumors.
Investigators at the University of Florida’s Brain Tumor Immunotherapy Program have leveraged the strengths of AAV gene delivery to develop a promising combination immunotherapy. The Powell Gene Therapy Center and the Clinical and Translational Science Institute at the University of Florida have been crucial in establishing the infrastructure and resources needed to pave the way for future viral gene therapies in GBM, providing clinicians with a powerful new tool to combat aggressive cancers.

Relevant Links
UF Brain Tumor Immunotherapy Program
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in