Acrasis kona: light version of aggregative multicellularity
Published in Protocols & Methods and Genetics & Genomics

We are surrounded by multicellular life, and it is the way of living we have been long accustomed for. Almost all the living world we see every day with the naked eye is multicellular, including animals, plants, even algae and fungi.
All these multicellular organisms have quite different development pathways. If the development of a cat and a human is similar, it is much harder to find similarities between the development processes in a fruit fly and a cat — of course, if you are not a developmental biologist. But even — accidentally! — if you have a degree in developmental biology, you will barely find any similarities in the embryonic development of a dandelion and human. These organisms are completely different.
But all of them share one important common trait: all of them start their development from a single cell. All cells of a rose or of a marine tubeworm are the descendants of a single cell, so the entire organism consists of a single cell clone. Thus, this way of living is called clonal multicellularity. What? There is another one? Yes!
Imagine the situation: single amoebas roam through soil and feed on bacteria that naturally occur there. They are their common food. But once the food resources are depleted, then the show starts. The amoebas produce a signalling compound, something like a pheromone, which attracts other amoebas. They form rivers of amoeba cells which stream to the single point where a mountain of amoebas grows. This mountain starts to behave as a single multicellular organism: it develops into a beautiful fungus-like structure or a structure resembling a tree. Then the hut of a “fungus” or the branches of a “tree” shiver into spores. They will be spread by the wind to more foodful places where they will grow to new amoebas.
Does it sound like a description of an alien world? But this world is literally under our feet. We often miss these organisms because their fruiting bodies are too small for us — to say nothing about the amoebas themselves. This is the world of aggregative multicellularity.
The most famous example of such lifestyle is Dictyostelium discoideum — a cellular slime mould and a useful model organism with tender, gracile, fungus-like fruiting bodies (Fig. 1). Its complex developmental cycle (Fig. 2) is driven by plenty of genes. DictyBase, the largest database of mutant Dictyostelium phenotypes, reveals that its development is disrupted upon damage of genes which have homologues in the human genome and control cell growth a differentiation. Dictyostelium is even used as a simple model to check developmental toxicity of drugs [1] — despite the fact that its aggregative development is completely dissimilar from developments of both cat and dandelion.

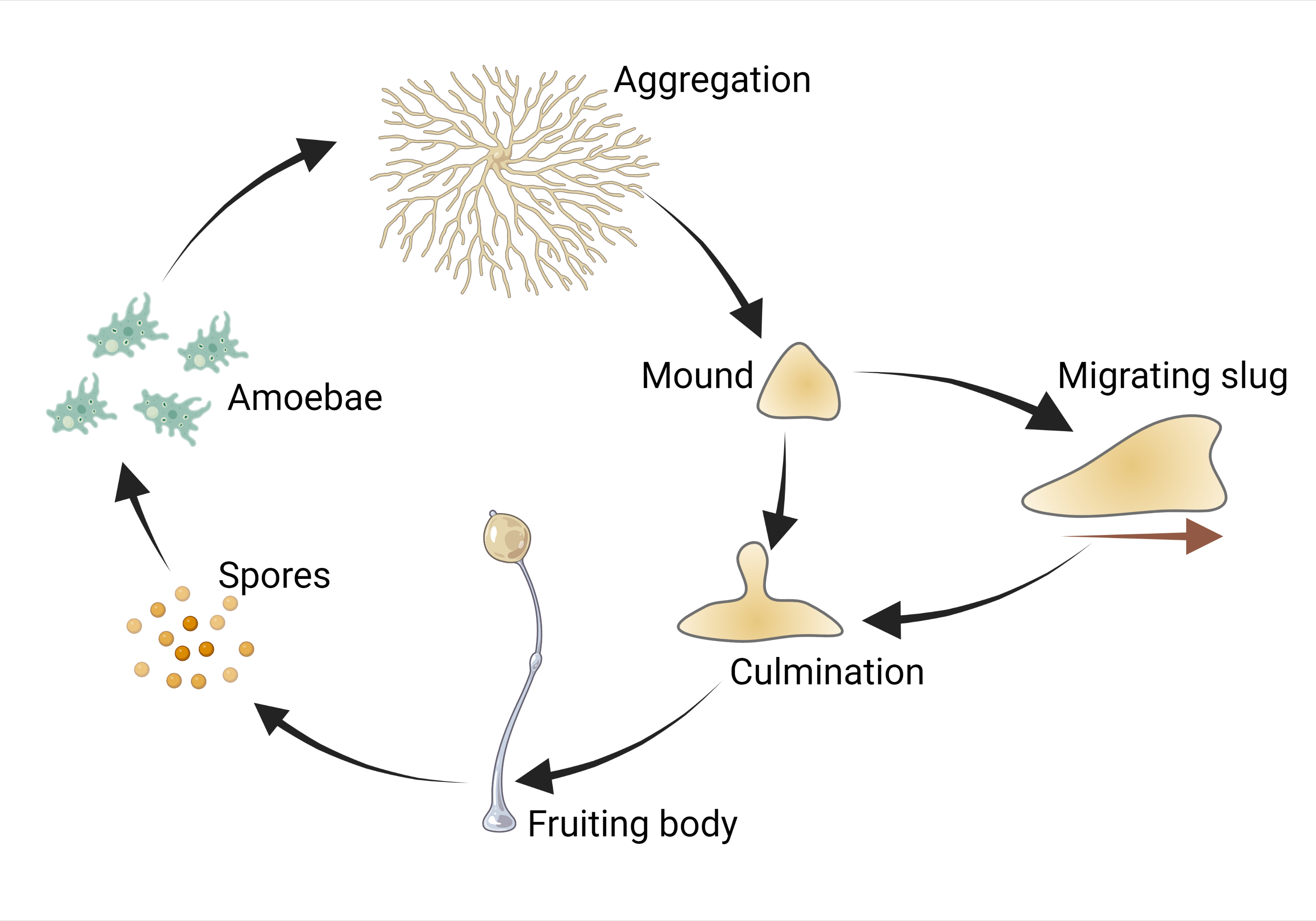
Phylogenetically, Dictyostelium is a close relative of fungi and animals, as well as of a plasmodial slime mould Physarum polycephalum, which is known for its ability to find the shortest ways and solve simple logical puzzles. The molecular basis of the dictyostelid multicellularity has been studied inside out — and this is an invaluable source of information on the evolution of multicellularity itself. But it is only one of the pieces of the aggregative multicellularity puzzle.
Media outlets have already disseminated the stories about Naegleria fowleri — a dreadful brain-eating amoeba that dwells in freshwater lakes. This deadly infectious agent belongs to the phylum Heterolobosea, where almost no multicellular forms occur. The crucial word is “almost” — naegleria has multicellular relatives that could rival dictyostelid slime moulds in terms of the beauty of the fruiting bodies. Meet acrasid slime moulds — other cellular slime moulds that didn’t share any common ancestor with dictyostelids and us since the time when LECA existed.
It would be extremely interesting to delve into molecular mechanisms of their aggregative multicellularity to compare them with those of dictyostelid slime moulds and possibly find universal genes and solutions for it. But there was just one thing: there was no available genome of Acrasis kona – the classical model representative of acrasids (Fig. 3). We could observe its complex life cycle resembling that of Dictyostelium discoideum — but had no idea which genes drive it.

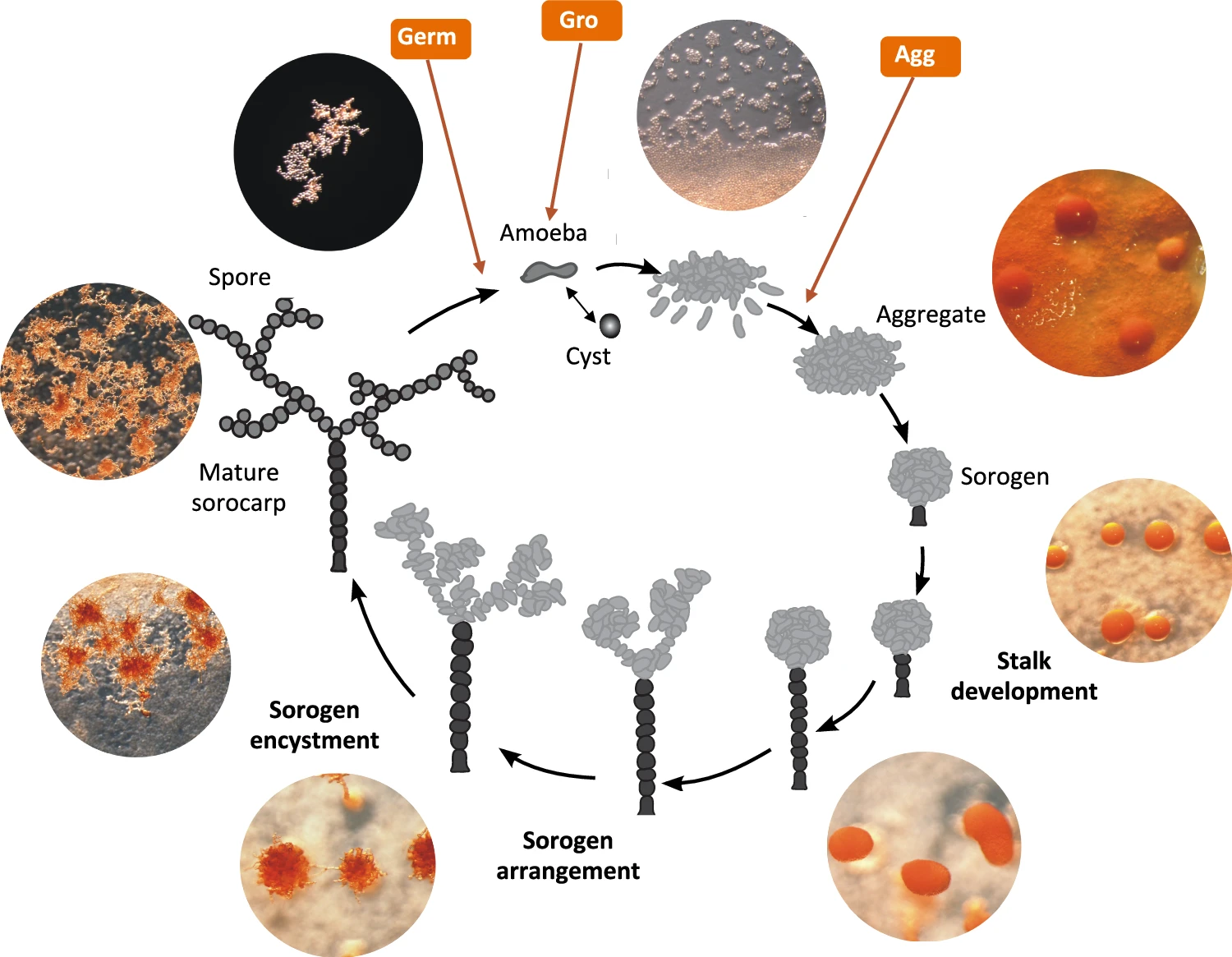
Things changed in the end of 2024, when a team led by Sandra Baldauf published the results of genome and transcriptome sequencing for Acrasis kona — a classical model acrasid slime mould [2]. Now we have its full genome sequence along with a predicted set of proteins.
Compared to their unicellular relatives, Acrasis kona appeared to have more genes and proteins related to the signalling process. Its genome is enriched with RAS GTPases, G-proteins, and components of phosphatidylinositol (PIP) signaling. These families power secondary messenger functions in a wide range of eukaryotic cells, including human ones. In this respect, Acrasis kona turned out to resemble humans.
The acrasid genome was also enriched for histidine kinases. This family of protein kinases exerts receptor and messenger functions and acts instead of tyrosine kinases in plants, fungi, and dictyostelid slime molds. This is a widespread family of signalling kinases in almost all multicellular eukaryotes — only metazoans use relatively unusual tyrosine kinases. Thus, there the acrasid is in line with other multicellular eukaryotes — where we are totally exotic.
Surprisingly, the genome of Acrasis kona appeared to contain genes of photoreceptor proteins presumably perceiving blue light. They are the members of the BLUF family (Blue Light receptors Using Flavine) – one of the six photoreceptor families known so far [3]. I would like to remind you that we use rhodopsins instead while plants rely on cryptochromes – by the way, this protein family also contains flavine as a light-sensing chromophore. Acrasids just seem to have employed one of the few light-sensing solutions widespread in bacteria.
Earlier, Acrasis kona had been characterized to respond to blue light by aggregation, which provides hints for the future searches of BLUF receptor functions in acrasids. Moreover, there is a fascinating parallel between BLUF in acrasids and another recent discovery published in Science Robotics [4]. A group of researchers from Cornell University made a simple analogue of Neuralink for a fungus to use its complex pattern of action potentials for driving robots. It is interesting that blue light was used here as a sensory input for a fungus – and fungus could perceive it directly and respond with electrical spikes. Together with the role of blue light in the sleep-wake cycle and its perception by plant cryptochromes, its universal role in regulatory processes could be a new promising research field. I assume it’s time to read the book Blue by Kai Kupferschmidt again…
Anyway, Acrasis kona seems to follow a common trend in the terms of perceiving blue light. From a genomic point of view, it uses the same molecular solutions for multicellularity as other multicellular eukaryotes use. However, transcriptomic analysis revealed how these genes function in the process of acrasid development — and showed some unusual things that we didn’t know earlier.
Aggregation of dictyostelid slime moulds — the most beautiful stage of their life cycle — is their swan song. It starts in the starvation conditions and is accompanied by active self-digestion of cells. Proteolysis and mitophagy feed the last processes in the slime mould’s life – construction of a stalk and sporulation – with substrates. But in Acrasis kona, researchers were surprised to find no transcriptomic trace of self-destruction upon aggregation. There was no up-regulation with autophagosome- and proteasome-related genes. RNAases — RNA-destructing enzymes – were not upregulated as well.
In contrast, Acrasis kona maintains a high level of expression of DNA replication proteins till the very end, when the spores form. Against the background of dictyostelids, Acrasis seems to be full of life upon aggregation. Transcriptomic trails suggest that the molecular mechanisms of its aggregation are strikingly similar to drivers of human cells signalling (Fig. 5) – as well cell signalling of other complex eukaryotes.
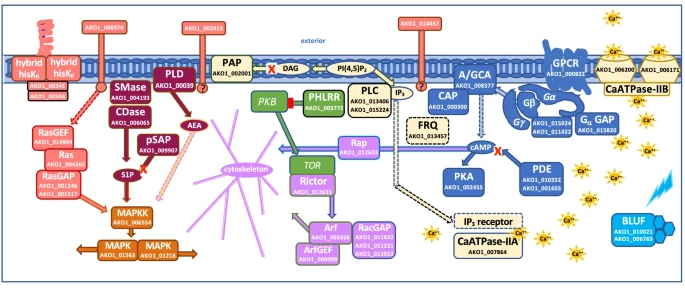
In particular, the expression of protein kinase A, the same protein kinase that mediates the action of epinephrine and kick-starts glycogen cleavage in humans, increases in acrasis during aggregation. Ras proteins, which regulate cell reproduction and differentiation in humans, and TOR protein, a homologue of mammalian target of rapamycin (mTOR, a promising target for the development of anti-aging drugs), also show a strong response to aggregation. Interestingly, acrasids “stole” one of the proteins of the phosphatidylinositol pathway from some fungus by horizontal gene transfer. And an intron splicing protein – from an unidentified dictyostelid slime mould! Indeed, why not borrow good technology from a competitor?
But the overall amount of genes involved was surprisingly low: only about 900 proteins change their expression upon aggregation of Acrasis kona, which corresponds to just 6% of all its proteins. In contrast, aggregation of Dictyostelium discoideum involves more than 4000 proteins, which account for 35% of all its proteins. From the transcriptome point of view, dictyostelid slime moulds look like a professional edition of aggregative multicellularity, while acrasids look like a lite version. They employ the multicellularity tools used by other eukaryotes, but the overall toolkit is minimal.
Thus, the authors have presented a first bird-eye view of the development of Acrasis kona. But they have also provided large sets of genomic and transcriptomic data, which had been long awaited by researchers.
I learned that the acrasid genome was sequenced at the ISCB-LATAM 2024 conference: Monica Gonzalez-Lazaro, a cellular biologist from Mexico, cited it in her poster when it was not published yet but presented as a preprint. Her work was dedicated to peroxisomes in Heterolobosea, and Acrasis kona genome appeared very timely for her. But the interest in the acrasid genome is not limited to heterolobosea studies: it has another significance.
I waited for this genome for almost 5 years, since I published the first work on my lipoxygenase project. I search for an evolutionary link between lipoxygenases and multicellularity, and any portion of genomic data on slime moulds is crucial for me. DictyBase shows that Dictyostelium discoideum does have a lipoxygenase, and its disruption leads to a developmental failure — more specifically, to the abolition of the culmination stage. But what about Acrasis kona – the slime mould with another design of aggregative multicellularity?
On this ISCB-LATAM evening, I failed to find a lipoxygenase in a newly published acrasid genome. But when I published a news article on the acrasid genome in Elementy – a Russian online science magazine – a user with the nickname “mol_biol” provided me with some hints in the comments section. It turned out that Acrasis kona has a putative lipoxygenase (UniProt ID A0AAW2Z831). According to the authors’ dataset, its expression shows no drastic development-driven changes (Gro v Agg = 0.75, Agg v Germ = 0.14). But the presence of this lipoxygenase itself is promising for new research on the connection of oxylipin signalling and multicellularity – even in completely different cellular slime moulds.
My colleague from the comment section also asked a question on whether integrins and adhesins are present in Acrasis kona or not. This led to a vivid discussion showing that the article in Nature Communications is not the final, but the beginning of the fascinating scientific story. In my magazine articles, I like to write that plants and animals have evolved completely different ways to be multicellular within the clonal framework. Now I can say the same about acrasids and dictyostelids – they represent completely different implementations of aggregative multicellularity on almost the same molecular basis. Thus, the new NCBI genome and Excel tables in the article’s supplements are not just data on Acrasis kona. They are data on a new way to be multicellular – and its full evaluation is still ahead.
References
- Baines, R. P., Wolton, K., & Thompson, C. R. (2021). Dictyostelium discoideum: An alternative nonanimal model for developmental toxicity testing. Toxicological Sciences, 183(2), 302-318. https://doi.org/10.1093/toxsci/kfab097
- Sheikh, S., Fu, C. J., Brown, M. W., & Baldauf, S. L. (2024). The Acrasis kona genome and developmental transcriptomes reveal deep origins of eukaryotic multicellular pathways. Nature Communications, 15(1), 10197. https://doi.org/10.1038/s41467-024-54029-z
- Jung, A., Domratcheva, T., Tarutina, M., Wu, Q., Ko, W. H., Shoeman, R. L., ... & Schlichting, I. (2005). Structure of a bacterial BLUF photoreceptor: insights into blue light-mediated signal transduction. Proceedings of the National Academy of Sciences, 102(35), 12350-12355. https://doi.org/10.1073/pnas.0500722102
- Mishra, A. K., Kim, J., Baghdadi, H., Johnson, B. R., Hodge, K. T., & Shepherd, R. F. (2024). Sensorimotor control of robots mediated by electrophysiological measurements of fungal mycelia. Science Robotics, 9(93), eadk8019. https://doi.org/10.1126/scirobotics.adk8019
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in