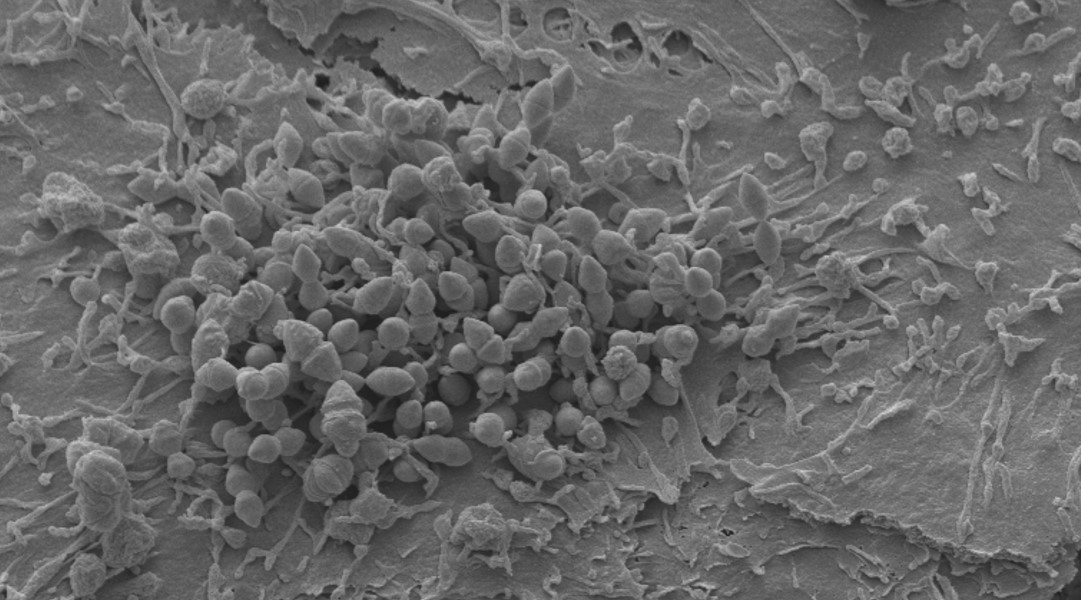
Human colonisation by Streptococcus pneumoniae is a prerequisite for transmission and pneumococcal disease. Our research considers the effects of Streptococcus pneumoniae infection at the level of the nasal epithelium. Our paper “Microinvasion by Streptococcus pneumoniae induces epithelial innate immunity during colonisation at the human mucosal surface” demonstrated pneumococcal micro-colony and micro-invasion of the epithelium. This was associated with epithelial transcriptomic and protein responses that coincided with bacterial clearance in a human challenge model (EHPC).
Results from the EHPC model led to a larger project collaboration between UCL and LSTM. The Brown lab at UCL generated mutants of the EHPC pneumococcal strain 6B. Using these strains, we can eliminate inter-serotype differences and tease out more precise effects from differing pneumococcal-epithelial interactions in human systems. I was successful in an internal UCL Innovation Postdoctoral Award to use these strains with our in vitro cell culture models and compare the adhesion, micro-invasion and transmigration of the strains, compared to wild type.
Following the results in our Nature Communications paper, we also hypothesized that the transcriptomic and protein epithelial responses would be different if the strains’ interactions with the epithelium were affected. I applied to the HIC-VAC society (co-funded by the MRC and BBSRC) to obtain funding for RNAseq analyses and cytokine/chemokine analyses from in vitro and in vivo infections and was awarded a Pump-Priming Grant as lead PI. This grant was pivotal in the collection of data from the cell culture model because, using our methods from the original paper, we were able to again dissect the contribution of the epithelial transcriptomic signature from the EHPC model. The results have revealed an unexpected phenotype that we are currently investigating.
As it is not possible to determine the contribution of different cell types to the cytokine/chemokine dataset obtained in the EHPC model, I successfully applied for an enhancement award from the HIC-VAC society and proposed a co-culture of primary epithelial nasal cells (a new collaboration with The Francis-Crick Institute) with/without innate immune cells. We will collect supernatant and apply Luminex technology (LSTM) to analyze the innate immune cell and epithelial cell secretions from cultures infected with the different pneumococcal strains.
Additionally, we are expanding our research to investigate epithelial sensing of the pneumococcus in more detail. Led by Professor Robert Heyderman, our successful MRC project grant application uses the models to investigate the intracellular signaling pathways activated during pneumococcal infection in healthy and vulnerable populations, such as the elderly.
In summary, the work we published in Nature Communications opened multiple avenues of research that we are currently exploring, as well as offering new opportunities for collaborations. We are excited to continue this trajectory and are now working towards a COVID study using the methods we have established.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in