
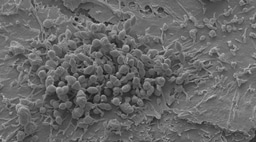
Colonisation of the nasal epithelium by Streptococcus pneumoniae (also called the pneumococcus) is a prerequisite for diseases such as pneumonia, meningitis and sepsis. A detailed understanding of the initial interactions that occur between pathogen and the human host are relatively unknown and difficult to study. Here, we were able to make use of a revolutionary Experimental Human Pneumococcal Challenge (EHPC) model where healthy human volunteers are inoculated with live bacteria and we are able study what happens at the mucosal surface. This is a safe procedure and the volunteers remain well. This model has allowed us to visualise, for the first time, pneumococcal colonisation of the nasal epithelium. We found that not only are these bacteria directly associated with the cells, but micro-invasion events (defined as internalisation of and transmigration across the epithelium) occur, in the absence of disease.
In this highly collaborative project, I was initially challenged to establish a working protocol for visualising colonisation in tiny tissue samples from the EHPC model. We spent time optimising the assay in vitro. Then, as the first volunteers of the study arrived, I processed the samples and tested the protocol for real. It took a total of 75 intense hours to manually scan all the microscope slides, searching for images to capture. There were some beautiful cell images, worth the patience (see below). Every single bacterium I found induced an adrenaline rush and was imaged for the record. The protocol also works for natural carriage and so opens avenues for exploring host-pathogen interactions in vivo, assessing markers of immune activation, identifying receptor-ligand interactions and investigating co-infection by different bacterial types. We then had to find a way to effectively study the host response to colonisation and have generated a novel RNAseq pipeline using transcriptomic data from in vitro infections experiments.
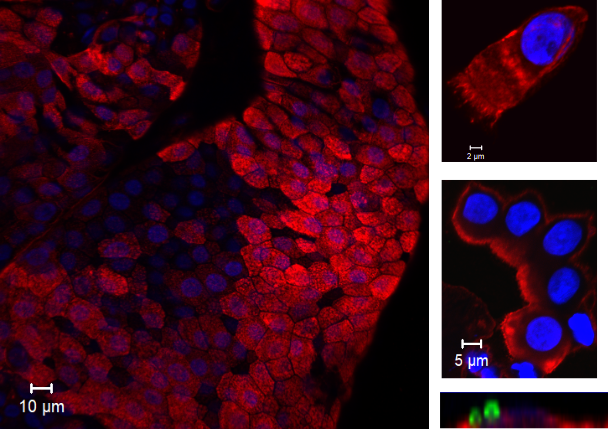
We hypothesised that these pneumococcal micro-invasion events trigger innate immunity by the epithelium that kick starts an immune response which may lead to clearance but could even assist with transmission. This seems counter-intuitive, but inflammation not only leads to bacterial killing but also increases production of nasal secretions which promote transmission on hands and by coughing. We think that some pneumococcal strains are more likely to micro-invade than others, this may lead to more inflammation, more rapid clearance but also transmission. Indeed, our in vitro RNAseq data confirmed that several epithelial signalling pathways are upregulated following infection with the pneumococcus, which was dependent upon the degree of micro-invasion with the epithelium. Furthermore, we have provided evidence to demonstrate that the activation of epithelial-innate immunity was highly influenced by the presence pneumolysin, a potent virulence factor that is known to induce inflammation.
Investigations into the mechanism and relevance of activating these signalling pathways in stimulating a protective immune response are currently our research focus. We are also studying bacteria modified in key virulence characteristics in the EHPC model. These experiments will provide clues as to how to optimise pneumococcal clearance from the nose and suggest novel targets for stimulating the immune system as part of vaccination.
Dr. Caroline Weight, UCL.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in