Aged hematopoietic stem cells entrap regulatory T cells to create a prosurvival microenvironment
Published in Cell & Molecular Biology
The problem we focused
Dysregulation of tissue-resident somatic stem cell homeostasis is widely considered as a key factor in the aging process. One well-studied example for investigating stem cell aging is hematopoietic stem cells (HSCs), as their aging significantly impacts the overall health span of systemic organs1-3. HSC aging is characterized by several changes, including diminished reconstitution capacity, expansion of myeloid-biased HSCs, increased replication stress, accumulated DNA mutations, enhanced resistance to apoptosis, impaired autophagy, and alterations in epigenetic, transcriptional, and translational profiles4. However, the precise interrelationships and hierarchical order of these aging characteristics in HSCs have yet to be fully elucidated. Particularly, the nature and timing of the initial events triggering HSC aging remain largely unknown, making it challenging to prevent HSC aging from an early age.
What did we show?
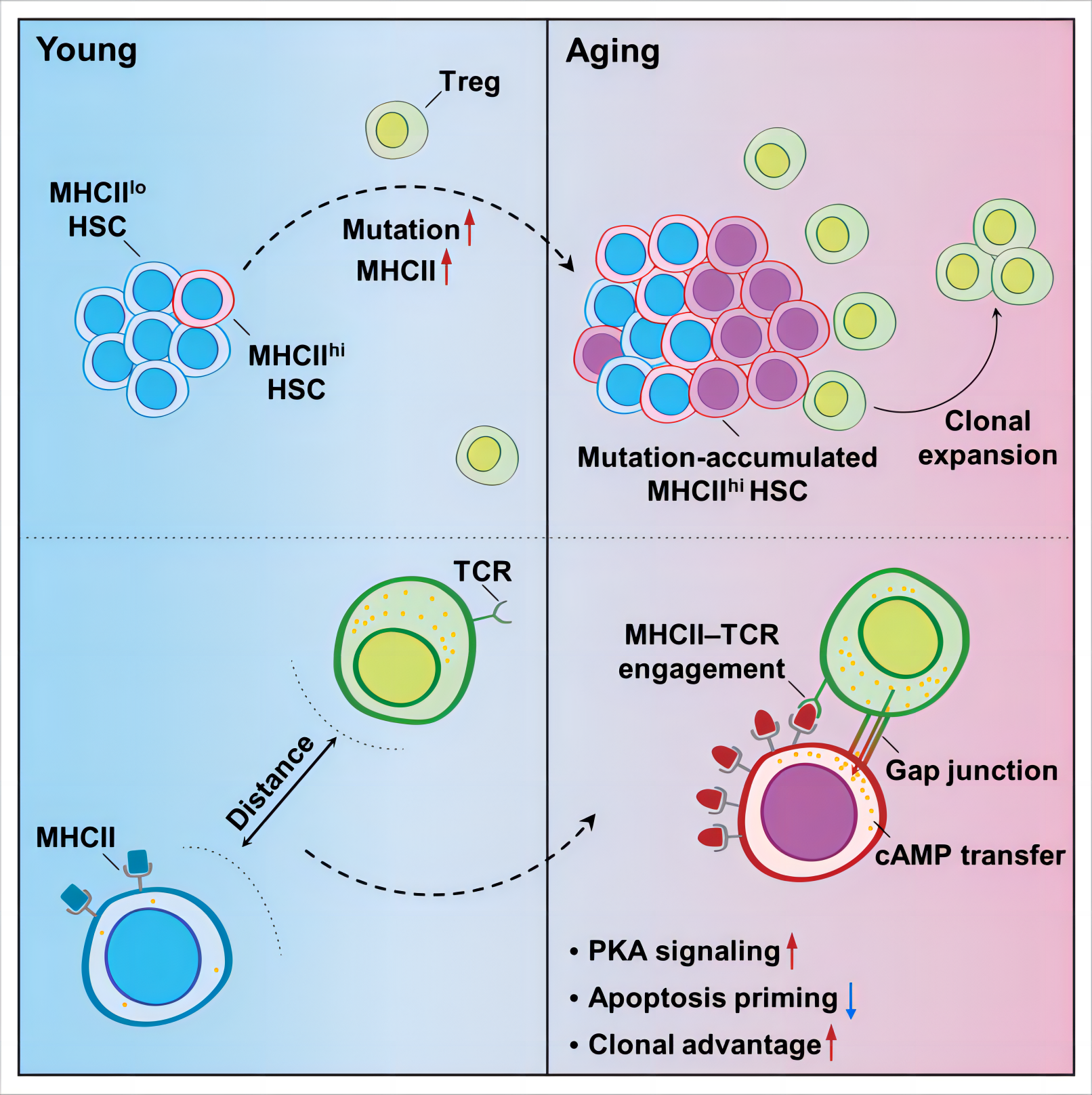
In this study, utilizing a mouse model of premature aging and middle-aged mice, we have identified and characterized a distinct role of bone marrow (BM) regulatory T cells (Tregs) in the process of hematopoietic stem cell (HSC) aging. Our findings reveal that the accumulation of DNA mutations in aged HSCs leads to an upregulation of major histocompatibility complex class II (MHCII) expression on HSCs, resulting in clonal expansion of BM Tregs. Furthermore, a reciprocal interaction is established between aged HSCs and BM Tregs through MHCII-T cell receptor (TCR) engagement. This interaction leads to the expansion of BM Tregs via TCR recognition of MHCII on aged HSCs, while simultaneously reducing the apoptotic priming of aged HSCs through the transfer of cyclic adenosine monophosphate (cAMP) from BM Tregs via gap junction (GJ) communication. Importantly, we have demonstrated that targeting the interaction between HSCs and Tregs can effectively prevent HSC aging starting from an early age.
Conclusions and implications of our study
The present study has unveiled an active and self-protective mechanism for aged HSCs to obtain clonal advantage by entrapping local Tregs to construct a pro-survival niche (Figure 1). Therefore, our findings present a promising strategy for preventing HSC aging by intervening aged HSC–Treg interaction.
You can access our publication here: https://www.nature.com/articles/s41423-023-01072-3
References:
1 Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100-105, doi:10.1038/s41586-021-03547-7 (2021).
2 Bogeska, R. et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29, 1273-1284.e1278, doi:https://doi.org/10.1016/j.stem.2022.06.012 (2022).
3 Jeon, O. H. et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nature Metabolism 4, 995-1006, doi:10.1038/s42255-022-00609-6 (2022).
4 Kovtonyuk, L. V. et al. IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood 139, 44-58, doi:10.1182/blood.2021011570 (2022).
Follow the Topic
-
Cellular & Molecular Immunology

A monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, covering both basic immunology research and clinical applications.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in