Allosteric Control: OGA’s Built-In Dimmer Switch
Published in Biomedical Research
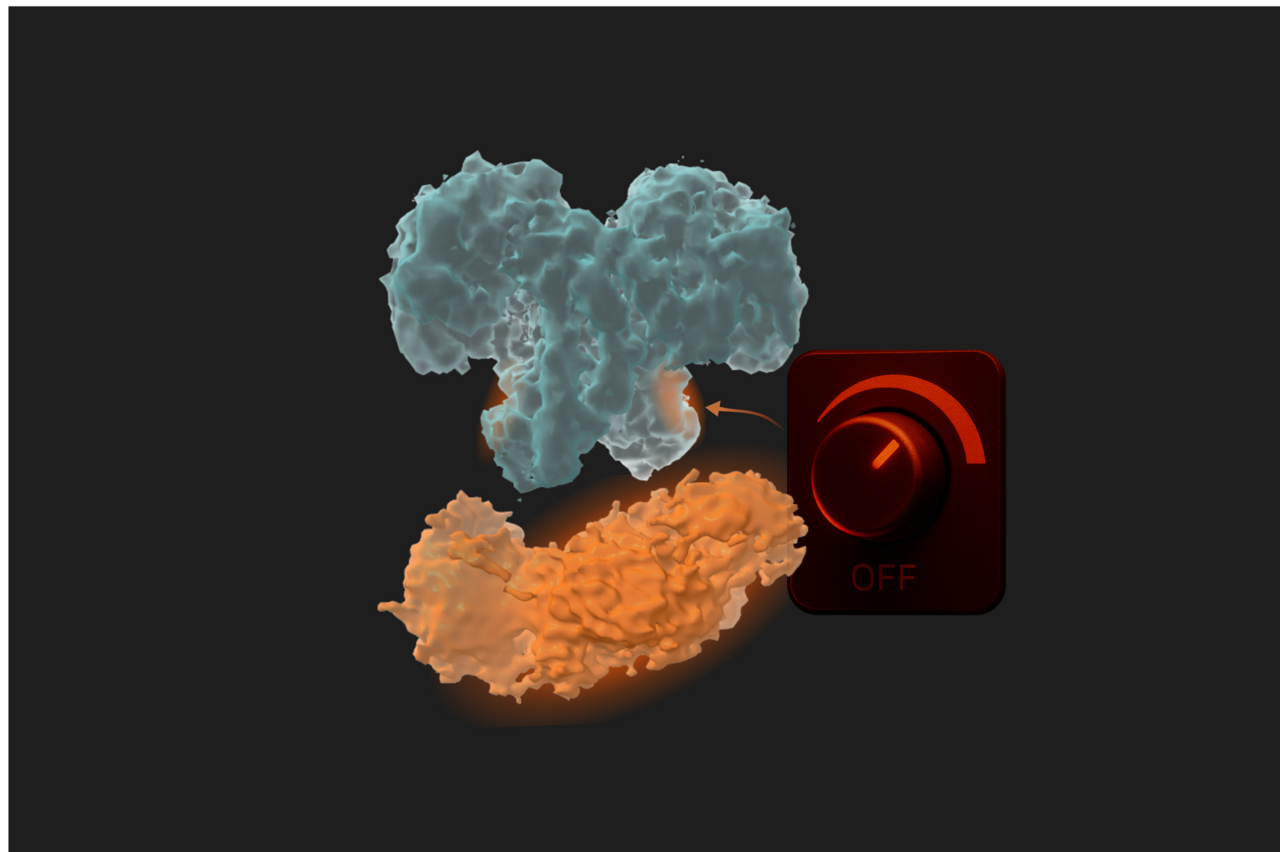
Because O-GlcNAc rapidly cycles on and off, even subtle changes in OGA activity can disturb cellular processes. Therefore, OGA malfunction has been linked to cancer, diabetes, and neurodegenerative diseases. Understanding how the entire OGA functions is crucial, leading us to investigate it’s not-so-well-understood extra domain.
What is the role of OGA’s additional domain?
Within OGA sits an additional domain that has puzzled researchers for years. On one hand, it has been related to the histone acetyltransferase (HAT) family of proteins. On the other hand, structural and biochemical evidence show that it is catalytically inactive, and it has since been referred to as a pseudo-HAT domain (pHAT). Thus, if it does not carry out chemistry, what could it be doing?
We reasoned that by looking at the full-length structure of OGA, we could begin to answer these questions. A complete structural picture would allow us to understand why it is not catalytically active, see how it is arranged, how it moves relative to the core, and whether these interactions could explain its role.
At first, the experimental data did not provide the clarity we hoped for. Indeed, the additional domain was so flexible that we couldn’t solve the complete structure at high resolution. Instead of the ordered atomic model we expected from our crystallographic experiments, we were instead confronted with cryogenic electron microscopy (cryo-EM) blurred density and missing pieces, exactly at the region we were interested in. This revealed that, despite being tethered to the catalytic core, this pHAT domain was not rigid at all. It was so unexpected that we needed to confirm it by small-angle X-ray scattering (SAXS) experiments.
That realisation raised a new set of questions. Why would evolution preserve a domain that behaves in this way? What function could such flexibility serve? And how could we make sense of structural data when we cannot see the protein parts we are interested in?
At this point, it was clear that the flexibility itself was a key finding. Thus, showing that this domain is mobile and dynamically linked to the catalytic core provides a foundation for future studies to build upon.
When the pieces started to fall into place
As we began writing up the manuscript and carried out the final rounds of cryo-EM refinements, something surprising happened: we realised we could now separate our cryo-EM particles into several subclasses. Suddenly, the blurred density started to make more sense.
This was a turning point. By resolving these subclasses, we could begin to see how the flexible domain behaves in different conformations, and the OGA “puzzle” started to make sense.
We could now see how the pHAT domain stabilised a flexible region within the catalytic core. This region directly shapes the environment leading to the active site where O-GlcNAc is removed from its substrates. It is therefore likely that the position of the pHAT domain influences the active site accessibility and thereby regulates both the activity and the substrate selectivity of OGA.
Armed with this new understanding, we were able to test our hypotheses directly by using a novel O-GlcNAc FACS sorting cell-based reporter system developed in our lab. These experiments gave us solid evidence that our previous thoughts about the pHAT role were correct. The pHAT domains are not just passive appendages, but active OGA allosteric regulators, which regulate OGA itself through a flexible linker region.
This also prompted us to revisit structural data from the simplest organism, Trichoplax adhaerens, an OGA ortholog that we had previously generated, but we had struggled to put into context. With our new insights, we were able to interpret this dataset more clearly and highlight differences in how OGA can be configured across organisms.
A new view of OGA
Until now, we did not know that the activity of OGA could be influenced by its own additional domains. We assumed that one domain was responsible for the catalytic activity, while the other was a localisation domain acting simultaneously but independently. However, our work shows otherwise: OGA carries a built-in control mechanism that might work as a dimmer-like switch on an enzyme we thought only had an on–off switch. In essence, this dimmer could add the extra regulation layer needed to interact with thousands of protein substrates.
This changes how we think about OGA, not as a simple machine, but as a finely tuned system where flexibility and regulation go hand in hand.
The implications go far beyond basic science. Future drugs might exploit this allosteric regulation to adjust OGA’s activity more precisely, sparing normal cellular functions, instead of shutting the enzyme down entirely.
And while many questions remain, uncovering this hidden layer of control opens up new directions for understanding cellular regulation and for imagining more selective and safer ways to intervene in disease.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in