Amazing protein evolution in Escherichia coli
Published in Microbiology

Microorganisms including bacteria can display vastly different shapes and sizes but can also alter shape and size as part of their natural development. Escherichia coli, a rod-shaped commensal bacterium of the human gut, readily alters its shape upon exposure to a variety of stresses. Upon many occasions such as exposure to certain antibiotics, but also upon escape from intracellular biofilms, E. coli stalls cell division, but still grows in length, which leads to the development of filamentous cells.
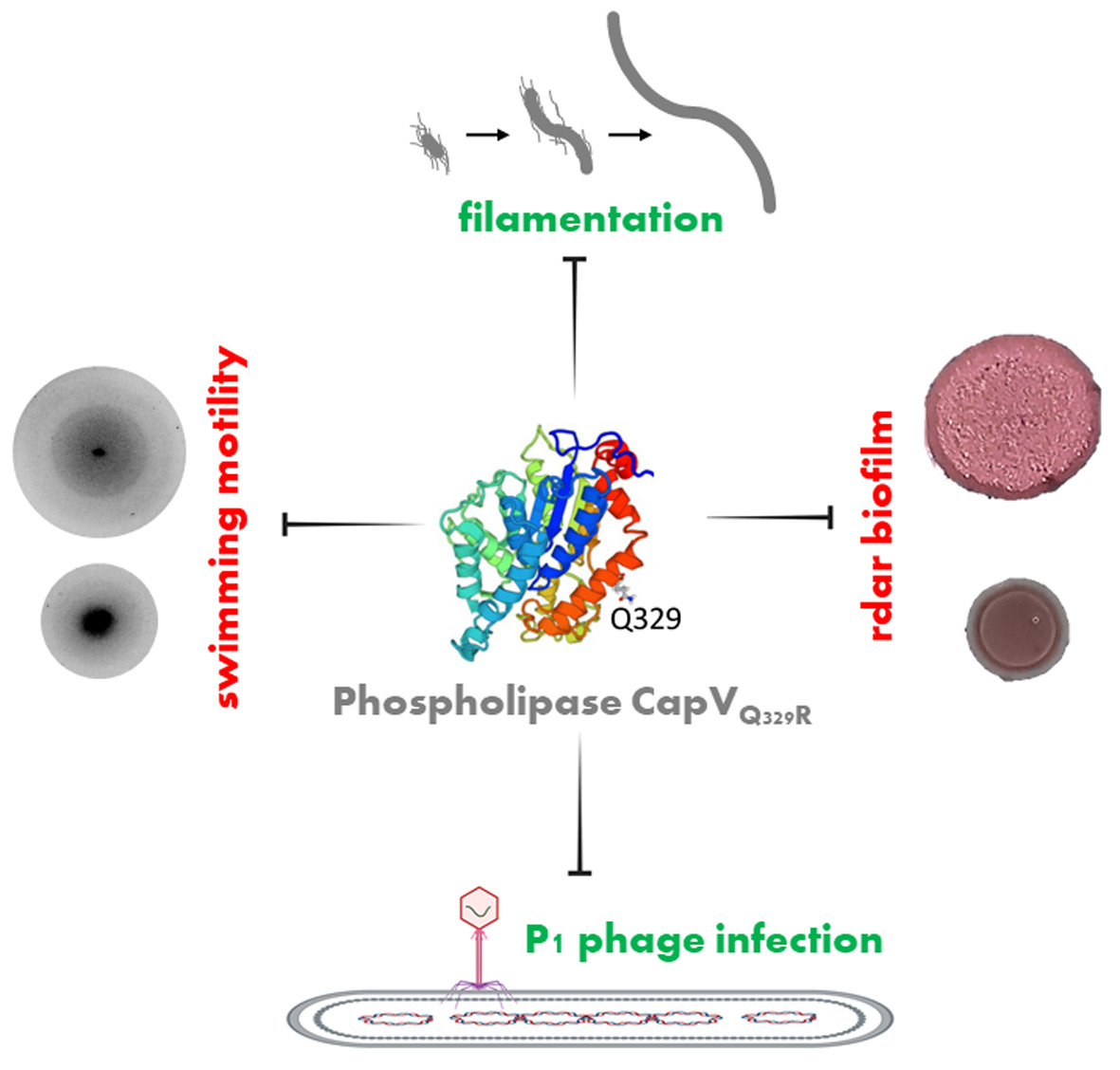
In our work, we discovered a single amino acid substitution in a horizontally transferred patatin-like phospholipase to dramatically manipulate various morphological and physiological functions in the model laboratory strain E. coli K-12 MG1655 in contrast to the wild type protein (Figure 1). As one of the most pronounced phenotypes, overexpression of the variant patatin-like phospholipase promoted extensive cell filamentation and concomitantly inhibited motility by early downregulation of flagellar appendages.
Phospholipases are enzymes that specifically hydrolize one or several (di)ester bonds of neutral lipids and phospholipids, major cell membrane components. Those enzymes participate in the turnover of the cell membrane, but can also be virulence factors of microorganisms. A particular class of phospholipases are the patatin-like phospholipases which occur in all domains of life. Patatin-like phospholipases received their name from patatin, which, in its major functionality, is an abundant storage protein of potato tubers. One might therefore ask the question why patatin-like phospholipases do not lyse the cells which express these enzymes. The answer is provided in the fact that the catalytic activity of patatin-like phospholipases, as other lipases and other degradative enzymes such as proteases, needs to be tightly regulated in time and space in order to be non-destructive. Only upon the presence of an activator signal, which might be a small molecule and upon interaction with another protein such as host ubiquitin, the catalytic activity is exerted. Patatin-like phospholipases are mainly known in eukaryotes to be involved in lipid homeostasis. In humans, the broad physiological impact of patatin-like phospholipases extends to, for example, energy metabolism and development of the skin barrier. On the other hand, patatin-like phospholipases can also be involved in second messenger signaling that includes hydrolysis of phospholipids to create lipid-based second messengers and development and maintenance of neuronal functionality.
Surprisingly, despite their fundamental role in lipid turnover, patatin-like phospholipases have rarely been investigated in Bacteria. As an almost exceptional example, the subject of this study, the patatin-like phospholipase CapV, has been originally identified as the receptor for the novel second messenger 3’,3’-cyclic AMP-GMP synthesized by the dinucleotide cyclase DncV. The capV-dncV gene cluster as part of a horizontally transferred signaling module is present in the current pandemic Vibrio cholerae El Tor clone, in animal and plant derived E. coli strains and the hospital acquired pathogen Klebsiella pneumoniae.
Additional effects of the expression of the Q329R variant of the patatin-like phospholipase CapV that had been observed included altered appearance of agar-grown colony morphotypes of bacteria. One of these morphotypes constitutes the rdar (red, dry, and rough) morphotype, which represents a highly conserved type of ecologically and clinically relevant biofilm formation characterized by amyloid fibers and the exopolysaccharide cellulose as extracellular matrix components. Expression of the variant patatin-like phospholipase repressed the rdar colony morphotype and promoted filamentation in other commensal and pathogenic E. coli strains genetically unrelated to E. coli K-12 MG1655. Further physiological alterations were observed in E. coli K-12 MG1655. Overexpression of the variant patatin-like phospholipase significantly enhanced the susceptibility to infection by the myophage P1 and to the β-lactam antibiotic cephalexin. Overall, these findings indicate substantial transformation of cell morphology and bacterial physiology by a variant patatin-like phospholipase. These significant alterations might aid to unravel novel regulatory mechanisms of cell division, biofilm formation and motility. Furthermore, the findings demonstrate rapid alteration in protein functionality by substitution of a single conserved amino acid in a protein derived from laboratory cultivated bacteria with the selection pressure being enhanced copy number by overexpression of the respective signaling operon. The structural alterations in the variant patatin-like phospholipase to conduct these changes need to be unraveled in future studies.
by Fengyang Li and Ute Römling
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: Feb 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in