Amino acids bind to phase-separating proteins and modulate biomolecular condensate stability and dynamics
How Amino Acids Fine-Tune the Liquid-Like Droplets Inside Cells
Cells are not just bags of molecules—they’re full of microscopic droplets called biomolecular condensates. These condensates normally form through a process known as liquid–liquid phase separation, where specific proteins and RNAs cluster into dense, liquid-like compartments without the need for membranes. They’re crucial for controlling cellular functions from gene expression to stress responses.
But what keeps these droplets stable, and what makes them dissolve? A new study in Nature Communications by Evan Spruijt, Xufeng Xu, and colleagues at Radboud University reveals that the answer may lie in the cell’s most basic ingredients: amino acids.
The overlooked role of amino acids
Amino acids are best known as the building blocks of proteins. These free amino acids also play a crucial role in influencing metabolism and maintaining osmotic balance. The researchers asked a deceptively simple question: Do amino acids interact directly with the proteins that make up condensates—and if so, how does that affect the condensates’ properties?
Glycine as a model molecule
To investigate, they started with glycine, the simplest amino acid. Using a well-studied model of the nucleolus—a condensate made of the protein nucleophosmin (NPM1) and ribosomal RNA—they observed how glycine affected the droplets’ formation.
As glycine concentration increased, the condensates became less dense and more dynamic. Quantitative imaging showed that NPM1 molecules inside the droplets decreased sixfold, while their concentration outside the droplets rose by 50%. In other words, glycine made the droplets “leak” protein, by weakening their cohesion. This effect further increased the dynamics and lowered the loading capacity of client molecules (Figure 1).
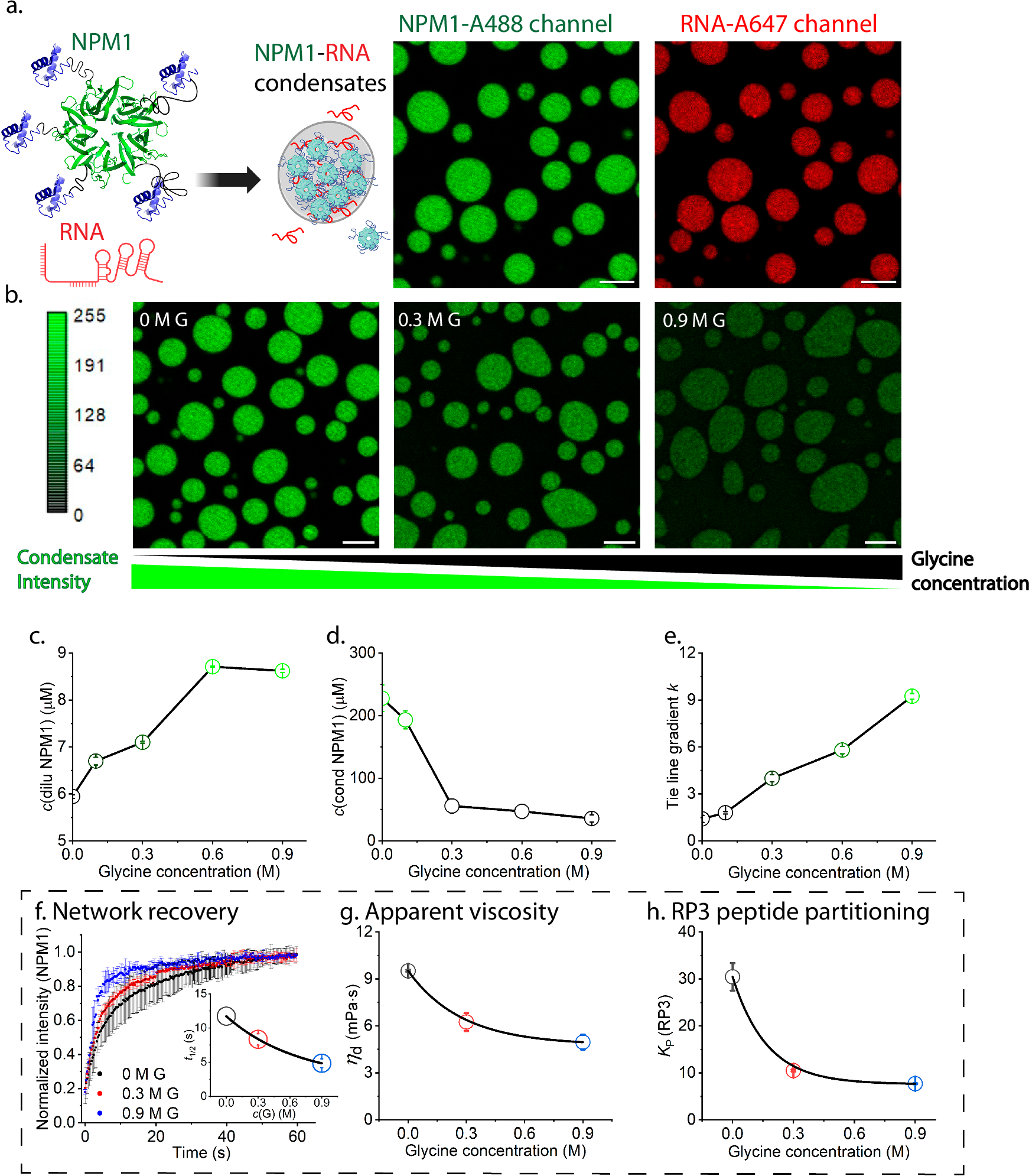
Figure 1: The phase behaviour and material properties of NPM1-RNA condensate after the addition of glycine.
Testing the rule across systems
To confirm this, Xu and colleagues tested a range of synthetic and natural condensate systems:
- Electrostatically driven condensates (K72–ATP and K10–D10 droplets) dissolved when amino acids were added.
- Condensates formed by aromatic interactions (WGR-4 and FFssFF) became more stable.
Significant effects from weak binding
To find out why, the team turned to nuclear magnetic resonance (NMR) spectroscopy. They discovered that glycine binds weakly—but specifically—to the amide groups in protein backbones and to aromatic side chains such as phenylalanine and tryptophan.
This weak binding interferes with hydrogen bonds in the protein backbone, which normally help hold protein chains together, while simultaneously strengthening certain π–π and cation–π interactions. The result is that condensates driven by electrostatic interactions tend to dissolve, while those stabilized by aromatic or π-type interactions are enhanced.
The apparent binding affinity of glycine is approximately 1 M, indicating a reversible, low-affinity interaction. Yet, because cells are rich in amino acids, even weak binding can collectively reshape the internal physics of condensates (Figure 2).
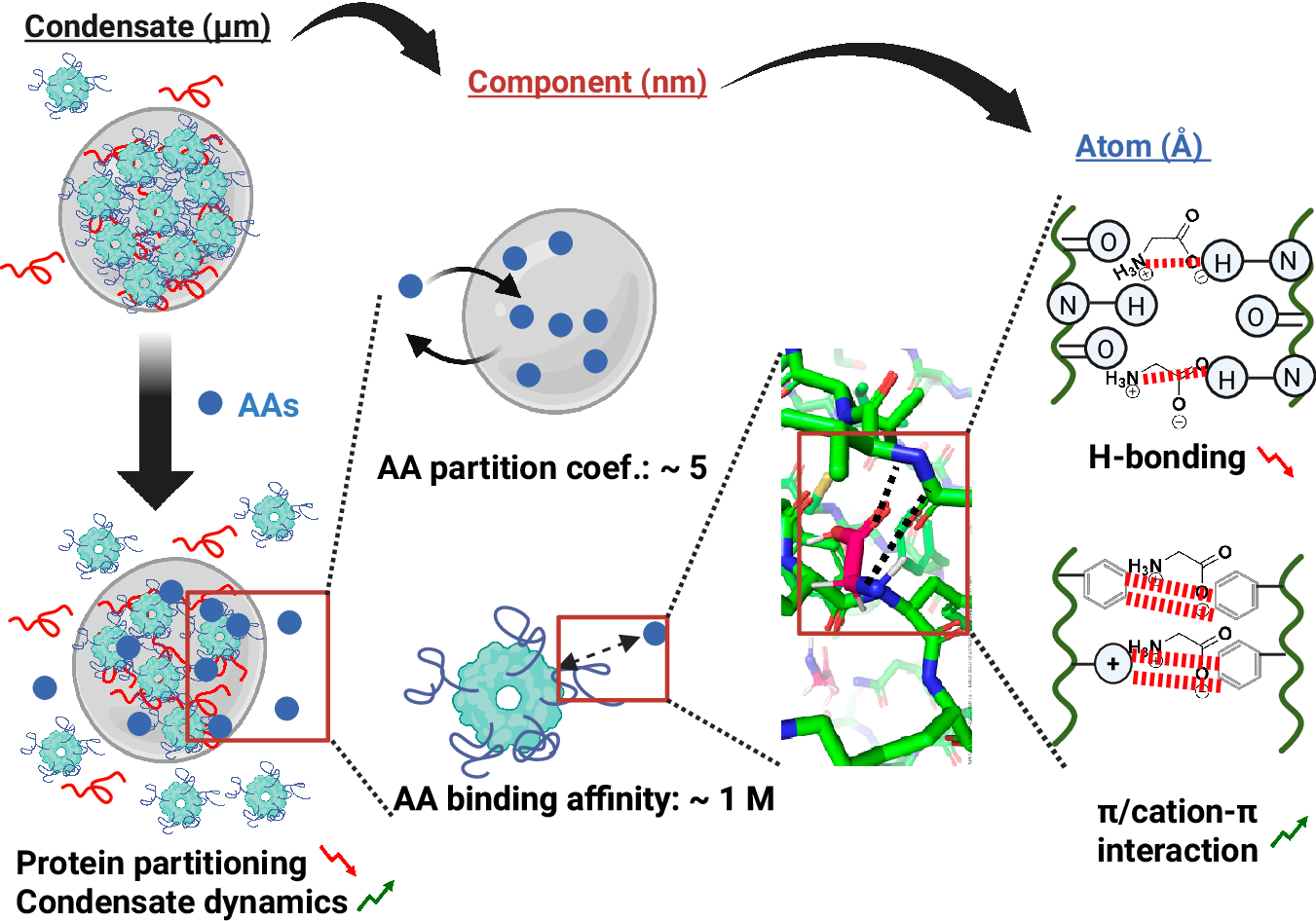
Figure 2: The proposed multiscale mechanism for the modulation effect of amino acids (AAs) on biomolecular condensates, ranging from µm-scale condensate formation and dynamics, nm-scale component interaction and partitioning to Å-scale atomic interaction.
Beyond glycine: a universal mechanism
Was this just glycine’s trick, or a general rule? The researchers screened all 20 proteinogenic amino acids using a high-throughput microplate assay. Nearly all had similar modulation effects. Even short peptides of two or more identical amino acids showed comparable behavior, implying that the effect is additive and could be engineered. This opens the door to designing small peptides that modulate condensate behavior in living cells, a potential route for treating diseases linked to aberrant protein phase separation and aggregation.
Why this matters
The discovery reframes our understanding of how intracellular chemistry governs cell organization. It suggests that condensates are not only shaped by macromolecules and ions but also by the chemical background of free metabolites, such as amino acids.
In health, such modulation could help cells to adapt to changes in nutrient availability or stress quickly. In disease, disruptions to amino acid levels—seen in metabolic syndromes or neurodegenerative diseases—might destabilize condensates, leading to protein aggregation and cellular dysfunction.
Beyond biology, this insight could inspire the development of new biomaterials, such as designer peptides that precisely tune phase behaviour for drug delivery, synthetic organelles, or bioengineering applications.
Reference:
Xu X., van Haren M.H.I., Smokers I.B.A., et al. (2025). Amino acids bind to phase-separating proteins and modulate biomolecular condensate stability and dynamics. Nature Communications, 16, 8723. https://doi.org/10.1038/s41467-025-63755-x
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in