An antifungal and its malcontents: Taming ‘ampho-terrible’
Published in Microbiology

“There are no solutions, only trade-offs.” – Thomas Sowell in A Conflict of Visions: Ideological Origins of Political Struggles
In terms of antibacterial agents, the development pipeline is getting rather constricted as major bacterial pathogens (notably Mycobacterium tuberculosis) have developed multiple drug resistance with time and often inappropriate antibiotic usage. Historically though, starting with penicillin, several antibacterials have been discovered and pressed into use over decades. The situation is very different with anti-fungal agents where the number of approved anti-fungals has persistently remained low, given that both fungi and their human host are eukaryotes, resulting in the challenge of identifying unique and pathogen-specific targets1. Antifungal development is thus beset with the problem of fewer candidates as compared to antibacterials, and even fewer success stories. This situation merits urgent attention, given that fungal infections are a particular cause for concern in immunosuppressed or immunocompromised individuals, as in transplant recipients or AIDS patients respectively. Readers will recall that, during the COVID-19 pandemic, secondary mucormycosis (‘black fungus’) infections posed a significant threat to patients in hospital intensive care units2.
Amphotericin B, a small molecule isolated from Streptomyces nodosus in 1955 at the Squibb Institute For Medical Research is effective against not only fungi but also protozoan pathogens, earning it a place in the WHO list of essential medicines. But disturbingly, it also exemplifies the cynical expression about the ‘cure being worse than the disease’ because of its side-effects, making treatment with it seem like a ‘trade-off’ rather than a ‘solution’.
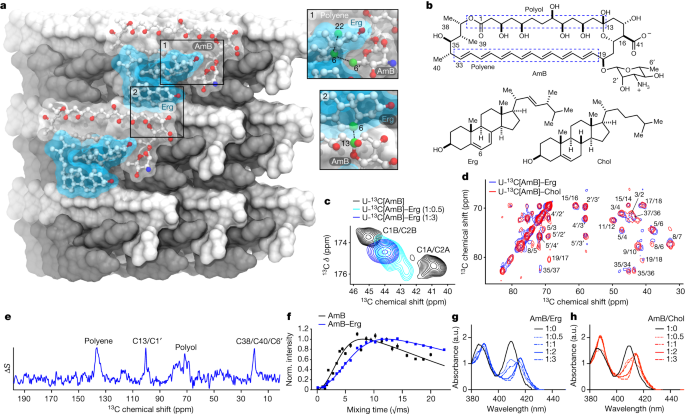
Amphotericin B binds sterols including ergosterol, a major component of fungal cell membranes, thereby hampering cellular processes, while also forming pores resulting in the rapid loss of cellular ions making it a very effective antifungal agent. In addition, it causes oxidative damage to cells via free radical formation, and stimulates phagocytes, promoting clearance of the infection3. At the molecular level, it forms extramembranous aggregates termed ‘sterol sponges’ that bind to and extract membrane sterols4. Unfortunately, its sterol-binding capability extends to cholesterol in mammalian cell membranes as well. The resultant side-effects that notably include kidney damage (nephrotoxicity) in majority of patients have severely limited its use.
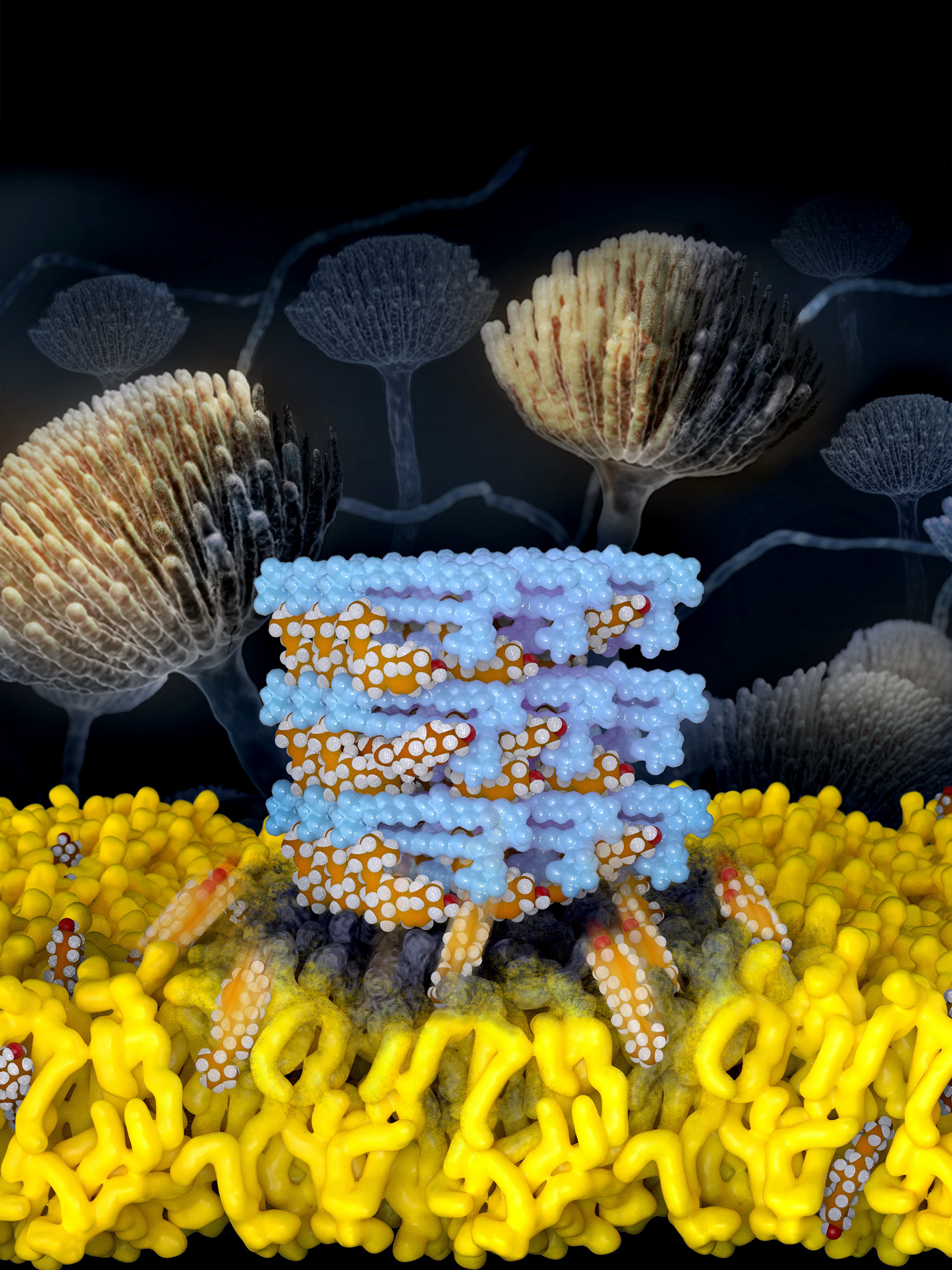
Having painted a rather dismal picture so far, we would like to make amends by highlighting recent research that offers a ray of hope. Maji et al.5 report their efforts in chemically modifying amphotericin B such that its antifungal effects remain relatively unaffected while its toxicity towards mammals is greatly attenuated. Of the several derivates synthesized, one termed AM-2-19 (see poster image on top), has been found to be distinctly promising. Firstly, it is significantly less mutagenic when tested in a bacterial reverse mutation assay as compared to the positive control compounds 2-nitrofluorene and benzo[a]pyrene and sodium azide, using bacterial strains recommended for the purpose by FDA guidelines. Secondly, it rapidly extracts ergosterol while sparing cholesterol. Thirdly, primary human renal proximal tubule epithelial cells (ATCC, PCS-400-010) and mice were found to tolerate the drug at much higher doses than used for amphotericin B. Finally, and most encouragingly in the context of antimicrobial resistance, not only is it active against several pathogenic fungi, but resistance development is remarkably rare.
This study brings to the fore the value of basic research – conventional wisdom about fungal killing by amphotericin B being primarily (rather than secondarily) due to pore formation had to be challenged first6. The sterol sponge mechanism of the fungicidal action by extraction of sterols was elucidated only in the 20144. Seemingly arcane details, the recognition of the sterol binding and extraction as the key mechanism underlying fungicidal action provided the impetus to rationally and selectively alter parts of the molecule such that it continued to extract ergosterol from fungal cell membranes at a high efficiency while attenuating its ability to affect cholesterol from host (human) cells. Also, not to be missed in this paper is the importance of maintaining good lab records – the name of the compound (AM-2-19) is a combination of the initials of the first author, his lab notebook 2 and page 19 therein. On the occasion of Antimicrobial Resistance Awareness Week (November 18-24, 2023), we sincerely hope this compound makes it to and through clinical trials.
Notes:
Poster image copied unchanged from supplementary information, section 2.4 of Maji et al5.
Disclaimer: The above post is not intended as medical advice. No guarantee is expressed or implied regarding the veracity and medical utility of the information provided on external websites and sources. The opinions expressed herein do not represent the views of the TERI School of Advanced Studies or TERI.
References:
- Bouz, G. & Doležal, M. Advances in Antifungal Drug Development: An Up-To-Date Mini Review. Pharmaceuticals (Basel) 14, 1312 (2021).
- Muthu, V., Rudramurthy, S. M., Chakrabarti, A. & Agarwal, R. Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World. Mycopathologia 186, 739–754 (2021).
- Noor, A. & Preuss, C. V. Amphotericin B. in StatPearls (StatPearls Publishing, 2023).
- Anderson, T. M. et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10, 400–406 (2014).
- Maji, A. et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature (2023) doi:10.1038/s41586-023-06710-4.
- Gray, K. C. et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109, 2234–2239 (2012).

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in