Motes in the eye: Gut microbiota breaching barriers
Published in Microbiology, Biomedical Research, and General & Internal Medicine
" And why beholdest thou the mote that is in thy brother's eye, but considerest not the beam that is in thine own eye?" – Matthew 7:3
Epithelial barrier integrity is of paramount importance for host organisms that harbor microbes in general, and for defense against pathogens in particular1. Even when the barriers are intact and functional, microbial cell components2 and metabolites3 do make it to the bloodstream. Additionally, some microbial metabolites are critical for the maintenance of barrier integrity4,5. If epithelial barriers are compromised, microbes as well as their metabolites and exogenous toxins can enter the circulation, causing serious infections as well as chronic conditions such as Crohn's disease.
The research paper by Peng et al. indicates how far intestinal bacteria can travel when epithelial barriers are compromised6. By using a careful combination of wild-type and mutant mice, both germ-free (GF) and specific pathogen-free (SPF) that were genetically ‘cured’, they demonstrated that intestinal bacteria may translocate from the gut all the way to the eyes. This was because these mice harbored a mutation in the Crumbs homolog 1 (CRB1) gene that adversely affects epithelial barrier integrity.
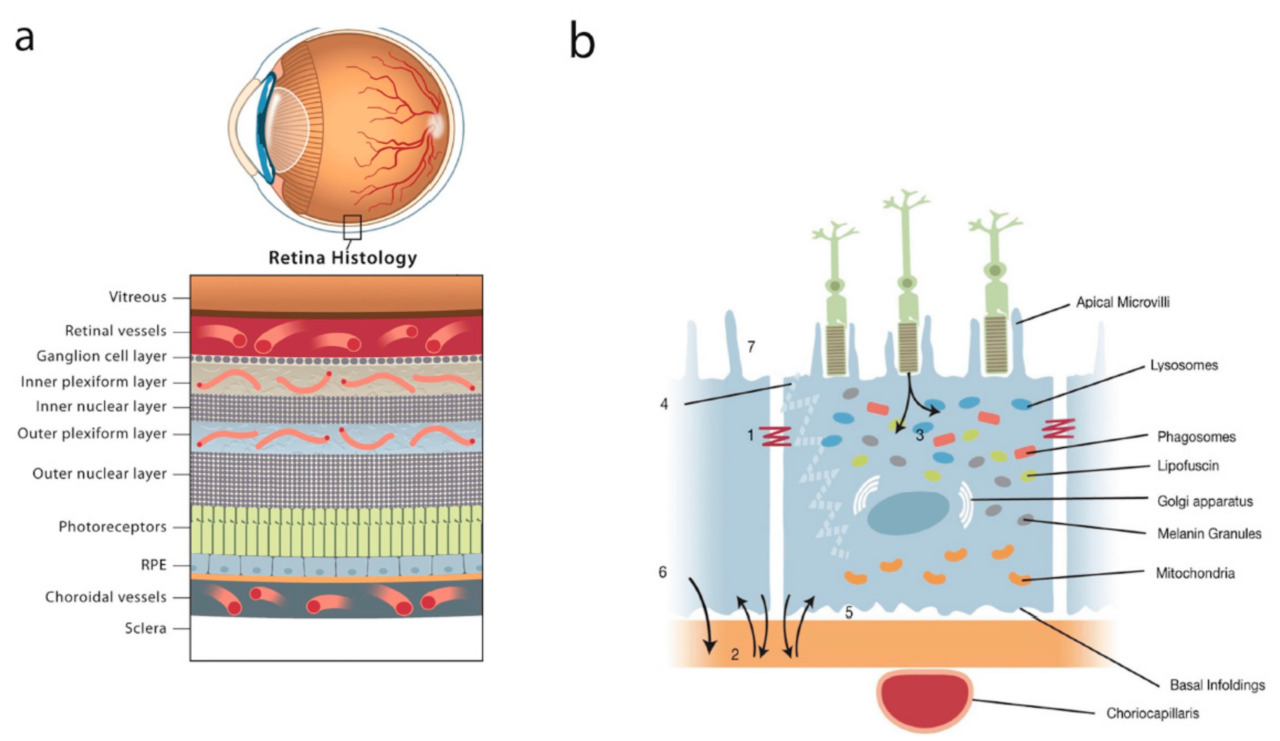
CRB1 is a protein conserved in both mice and humans and is expressed in the retinal pigment epithelium (RPE) and the brain. Mutations in the CRB1 gene are associated with retinal degeneration in human congenital diseases such as Leber congenital amaurosis and retinitis pigmentosa. CRB1 is a transmembrane protein that is critical for maintaining the apico-basal polarity of retinal epithelium as well as the integrity of adherens junctions. Mice harboring the naturally occurring Rd8 (retinal degeneration 8) mutation closely mimic the human disease and exhibit variable, scattered retinal lesions leading to progressive loss of photoreceptors, and hence blindness. This resembles the pathology seen in Crb1-/- mice.
But fate is not dictated by genes, for the environment is a major factor too. Comparisons of retinal histology in Rd8 and Crb1wt/wt (wild-type) mice that were maintained under SPF conditions indicated that only a few retinas of the mutant mice exhibited minor dysplasia during the embryonic stages. However, by post-natal day 12, all the mutant mice had retinal abnormalities, indicating that some environmental factors were at play. Accordingly, the researchers attempted to identify differentially expressed genes in the mutant mice as compared to wild-type using transcriptomics. A subset of these genes could be functionally categorized as being involved in ‘recognition of bacteria and viruses’ and ‘pro-inflammatory’ functions. This finding, coupled with the observation that mutant retinas exhibited infiltration by IBA1+ microglia/macrophages but not CD4+ T cells strongly suggested the activation of the innate arm of the immune system to pathogen invasion. Following up with metatranscriptomic and microscopy confirmed the presence of bacterial, but not viral or fungal species. Five out of seven bacterial species so identified occur naturally in the lower gastrointestinal tract and caecum of both mutant and wild-type mice. Thus, exposure to micro-organisms after birth during the course of microbiome assembly progressively exacerbates the retinal pathology of Rd8 mutant mice, and the bacterial offenders are gastrointestinal microbiota that find their way into the eye, which is normally an immunologically privileged organ.
But then, it is pertinent to ask if bacterial presence in the eyes is the cause or consequence or retinal degeneration. Pursuing this line of investigation, the researchers found that mutant mice raised under germ-free conditions did not develop retinal lesions, but did develop them once they were moved to specific pathogen-free conditions, indicating that bacterial colonization was the key determinant of retinal pathology. Furthermore, a dose of antibiotics also largely staved off lesions in mutant mice even under specific pathogen-free conditions.
Another key finding in this paper was that CRB1 is expressed not only in the eye and brain of mice and humans, but also on the apical surface of the enterocytes lining the colon, but not the caecum. Thus, when mutant mice receive gene therapy restoring CRB1 expression specifically in the gut, but not in other tissues such as the liver, spleen or retina, bacterial translocation and retinal lesions were significantly reduced. One might even predict that restoration of barrier integrity in both the intestine and the retina would have reduced the incidence of retinal lesions to an even greater extent, if not eliminated them altogether. After all, two successive barriers would be expected to have compounding effects as is the case with face masks in the context of respiratory diseases (notably COVID-19)7. In conclusion, this research is a fine example of elucidating mechanisms involved in the interaction of the microbiome with its host and the dependence of such interactions on host genetics. It demonstrates that ectopic colonization by otherwise innocuous bacteria can lead to severe pathologies in genetically predisposed hosts. Also notable in this paper are the extensive histological investigations that serve as milestones in pleasing lockstep with each successive mechanistic insight gained through the course of the study.
References:
- Panwar, S., Sharma, S. & Tripathi, P. Role of Barrier Integrity and Dysfunctions in Maintaining the Healthy Gut and Their Health Outcomes. Front. Physiol. 12, 715611 (2021).
- Wheeler, R. et al. Microbiota-induced active translocation of peptidoglycan across the intestinal barrier dictates its within-host dissemination. Proc. Natl. Acad. Sci. 120, e2209936120 (2023).
- Dekkers, K. F. et al. An online atlas of human plasma metabolite signatures of gut microbiome composition. Nat. Commun. 13, 5370 (2022).
- Hu, J. et al. Gut microbiota-derived 3-phenylpropionic acid promotes intestinal epithelial barrier function via AhR signaling. Microbiome 11, 102 (2023).
- Scott, S. A., Fu, J. & Chang, P. V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. 117, 19376–19387 (2020).
- Peng, S. et al. CRB1-associated retinal degeneration is dependent on bacterial translocation from the gut. Cell 187, 1387-1401.e13 (2024).
- Kollepara, P. K., Siegenfeld, A. F., Taleb, N. N. & Bar-Yam, Y. Unmasking the mask studies: why the effectiveness of surgical masks in preventing respiratory infections has been underestimated. J. Travel Med. 28, taab144 (2021).
Notes:
Poster image reproduced from Naylor et. al. (2020) (https://www.mdpi.com/1422-0067/21/1/211) under the Creative Commons Attribution License 4.0 (CC BY).
Disclaimer: The above post is not medical advice. No guarantee is expressed or implied regarding the veracity and medical utility of the information provided on external websites and sources. The opinions expressed herein do not represent the views of the TERI School of Advanced Studies or TERI.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in