An Unexpected Journey in Hybrid Adaptation
Published in Ecology & Evolution and Genetics & Genomics

Background
Hybridization is an intriguing phenomenon that can rapidly create new genotypes, often resulting in the emergence of unique traits. Research—both empirical and theoretical—has demonstrated that hybridization can drive swift evolutionary changes, particularly when facing adverse conditions 1, by generating greater genetic diversity than mutations alone, acting as a form of large-scale macromutation 2. Hybridization is part of our own story too! Humans carry genetic traces of hybridization with Neanderthals, which have shaped our genetic diversity and contributed to adaptive traits that help us thrive in various environments, such as immune responses and skin pigmentation 3. In the realm of yeast, hybridization has its historical roots at the Carlsberg Brewery (Denmark), where Emil Christian Hansen isolated the first hybrid yeast in 1883. While Hansen himself was unaware of its hybrid nature, this pivotal discovery laid the groundwork for understanding how hybrids can flourish under stress, and their use in fermenting wine and beer has since grown significantly in the industry. Yeast hybrids have also been documented in nature 4, showcasing the ongoing relevance of hybridization in both natural and industrial contexts.
The Double-Edged Sword of Hybrid Genetics
While hybridization can give rise to new traits, it also presents certain challenges, such as genomic instability. The combination of genetic material from two species complicates the interpretation and execution of genetic instructions, making the hybrid's genome more prone to mutations and chromosomal rearrangements, which can disrupt normal cellular functions.
Motivated by these factors, we previously used Saccharomyces cerevisiae and Saccharomyces paradoxus hybrids to test their ability to adapt to a drug that induces genomic instability, simulating UV exposure. After 100 generations of experimental evolution, we found that hybrids show a reduced adaptation compared to their parents (Figure 1) 5.
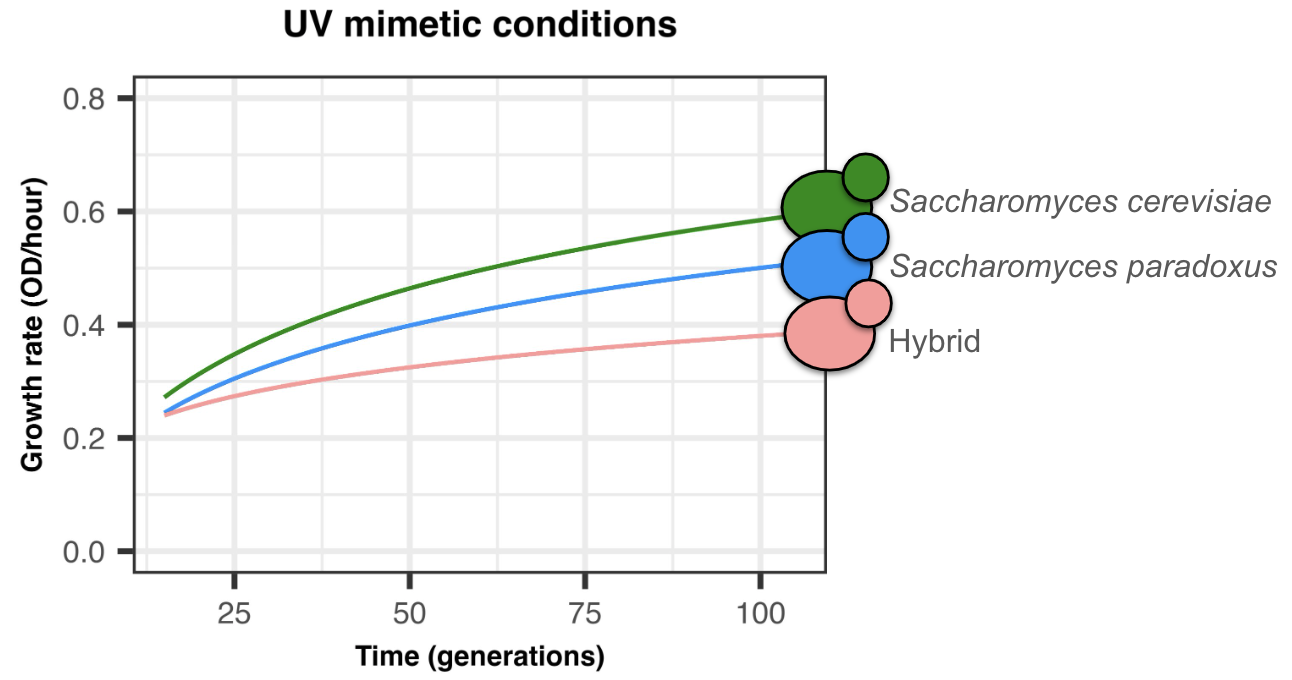
Intrigued by the hybrids' lower adaptation, we set out to uncover the mechanisms at play. We first hypothesized that their inherent genomic instability might impede adaptation by exacerbating genetic damage from the UV mimetic drug. We anticipated observing more genomic changes in hybrid populations, like ploidy alterations and aneuploidies.
Surprisingly, the hybrids showed a similar number of genomic changes as their parental species. This unexpected result left us questioning: if not genomic instability, what was slowing hybrid adaptation? I must admit that, at this point, we felt a bit of vertigo as our initial hypothesis quickly faded away. Nevertheless, we pressed on, as is well known in research: there are no bad hypotheses, only hypotheses that are disproven.
The Quest for Answers
With a renewed focus, we considered the possibility that hybrids may adapt through different mutations than the parental species, which could result in slowing down their adaptation. We identified mutations in the same genes that were under selection in both the parental strains and the hybrid, specifically in the genes PDR1 and YRR1. In fact, we found an adaptive cluster of mutations in which the parallelism we observed was astonishing! (Figure 2). Surprisingly, we also discovered this same cluster in other species, including the pathogen Nakaseomyces glabratus.
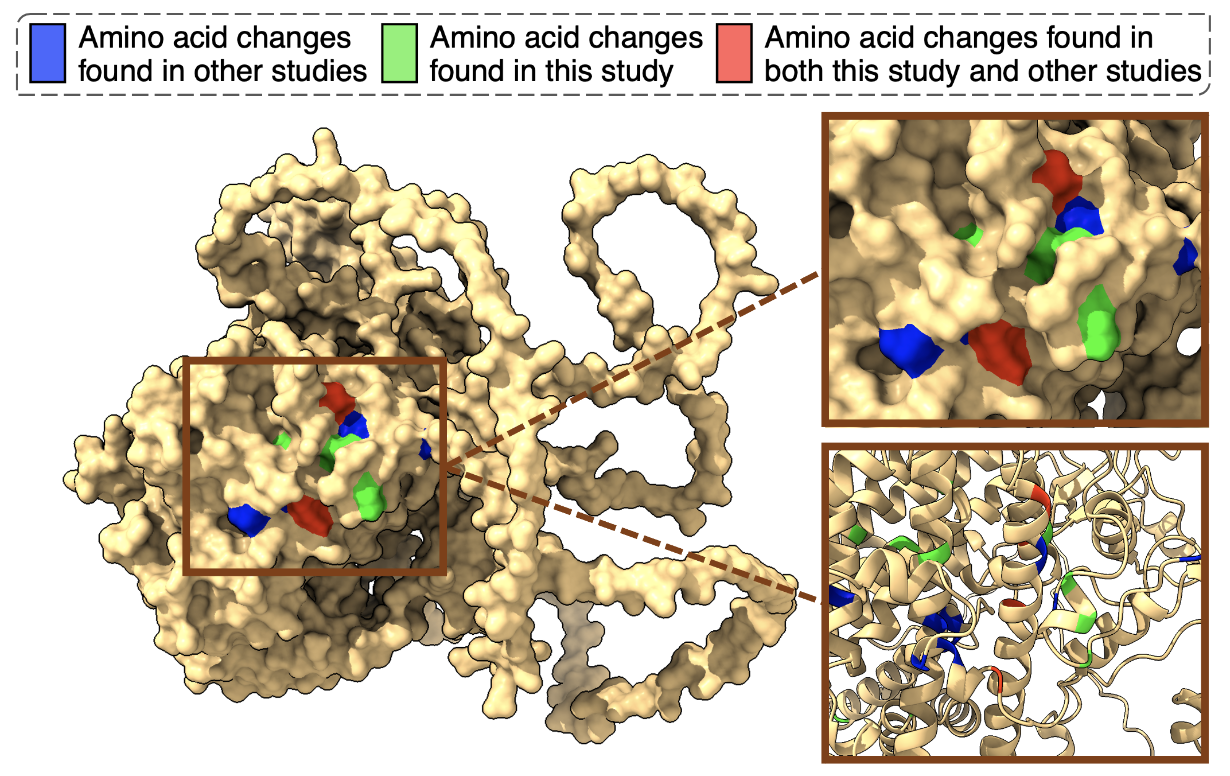
These genes are linked to resistance to multiple drugs and play a role in how cells expel toxic compounds (Figure 2), indicating that both hybrids and parents were employing similar molecular strategies for adapting to the UV mimetic drug. However, an even more puzzling question arose: why didn’t the same mutations confer the same level of resistance to both parents and hybrids? Things were getting increasingly intriguing…
Just when we thought we were more lost than ever, we uncovered the critical difference: mutations in these genes were predominantly homozygous (two copies) in parents, while they remained heterozygous (one copy) in hybrids.
Unraveling Haldane’s Sieve: The Role of Loss of Heterozygosity in Dominance Dynamics
At this point, I had to revisit my biology classes and refresh my understanding of genetic dominance to fully grasp what was happening. For an adaptive mutation to be beneficial, it must not only occur but also be in the right genetic context. In diploid organisms, dominant mutations spread more easily as they require only one copy to provide an advantage, unlike recessive mutations, which require two copies. This principle, known as Haldane's sieve, was proposed by J.B.S. Haldane, a key figure in the modern synthesis of evolution. Using genome editing techniques like CRISPR, we found that having two copies of a mutation was more beneficial than just one, meaning the mutations needed to be homozygous (two identical copies) to fully express their advantages.
In asexual populations like the yeast used in our experiments, mutations can bypass Haldane's sieve and become established in the population through Loss of Heterozygosity (LOH). This process occurs when a mutation in one chromosome copy is replicated in the other, creating two identical copies and rendering the mutation homozygous. However, in hybrids, differences between chromosome copies complicate the LOH, making it less likely to occur.
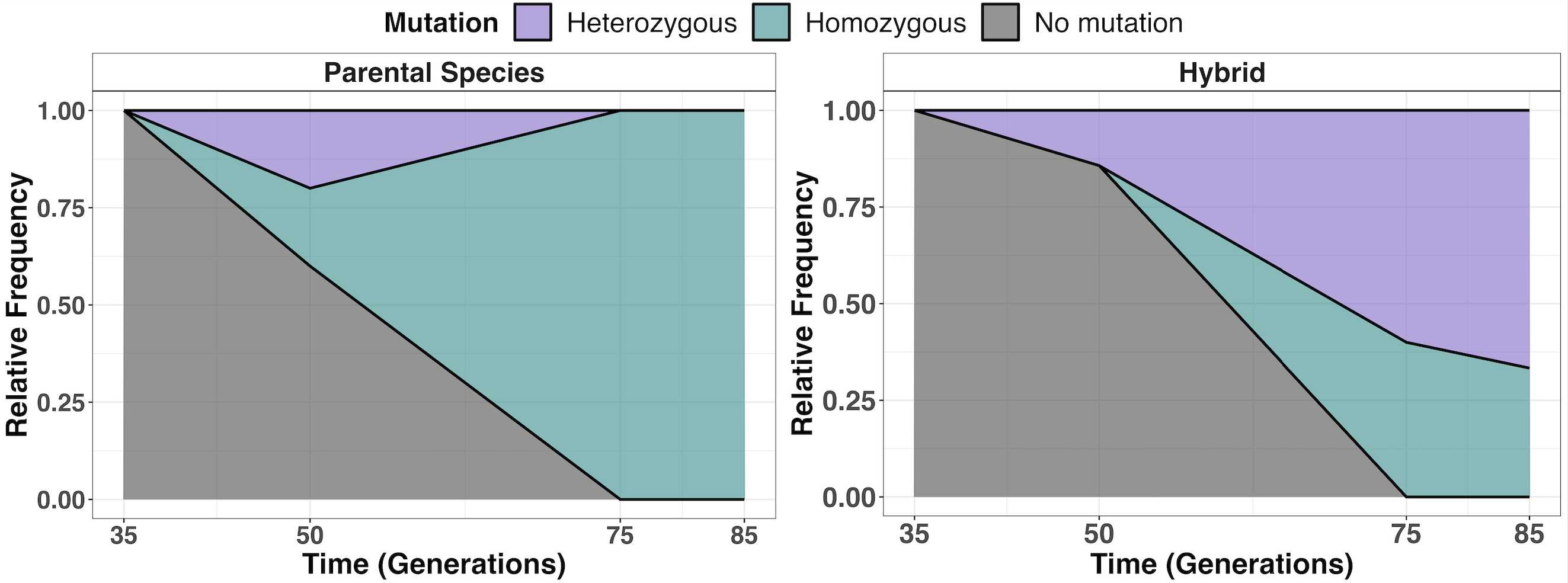
We hypothesized that the lower LOH rate in hybrids might limit their adaptation. To test this, we adopted a 'time machine' approach, analyzing our frozen populations to determine whether LOH occurred faster in parents than in hybrids. We tracked how many parental and hybrid individuals converted their mutations to a homozygous state over time. Our results showed a significantly higher proportion of homozygotes (two copies of the mutation) in parental strains compared to hybrids, confirming that LOH occurs at a slower pace in hybrids (Figure 3).
Final Reflections
Throughout this research journey, we have explored the fascinating dynamics of yeast hybrids and their unique adaptive pathways. Although we initially assumed that genomic instability might explain their lower adaptive potential, our findings led us to a more nuanced understanding of the underlying evolutionary mechanisms. We discovered that adaptation to UV mimetic conditions often proceeds through two primary types of mutations: nucleotide substitutions and LOH, with the latter occurring more slowly in hybrids and thus hindering their adaptation rate (Figure 4).
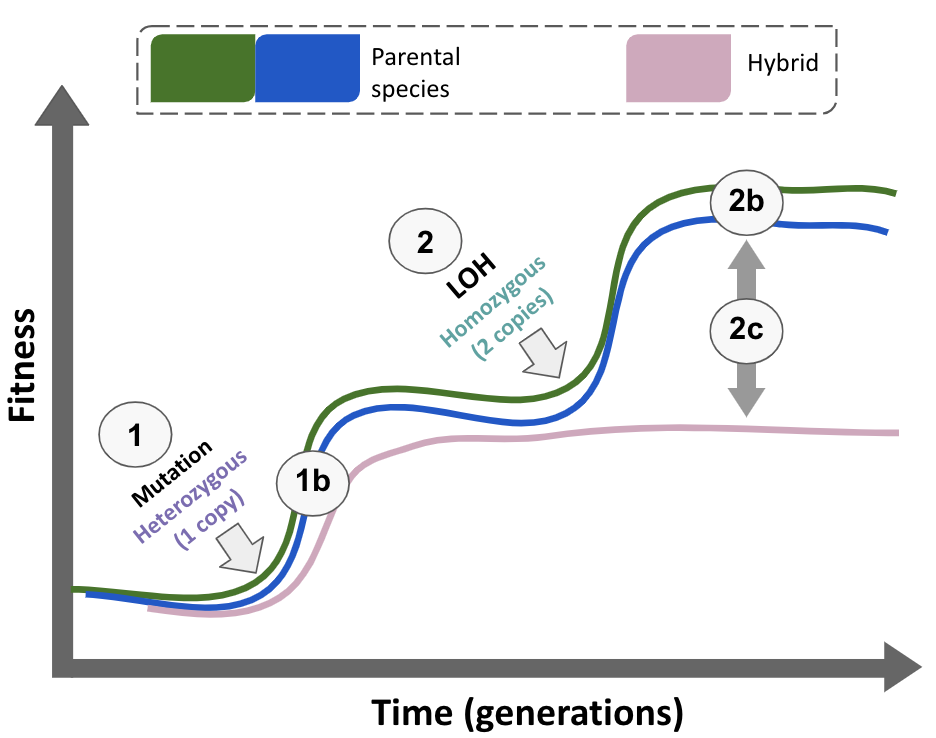
Now, the question is: what’s the purpose of all this? It’s important to clarify that for evolutionary biologists focused on fundamental research, uncovering adaptive patterns that shape the living world is inherently fascinating and satisfying in itself. However, our research could also provide valuable insights in three areas: improving hybrid strains for biotechnology, enhancing understanding of antifungal resistance through LOH in pathogens, and potentially slowing LOH to help control cancer progression.
This experience has deepened our understanding of genetics and evolution, highlighting the value of an open mind and adaptability in science. One key takeaway is that hypotheses evolve throughout research, and disproving them is a vital part of the scientific process. Science is a continuous journey, and each discovery brings us closer to understanding life’s mysteries. We are excited about the future of this research field and hope our findings inspire further exploration of the intricate pathways of adaptive evolution.
References
- Lewontin, R. C. & Birch, L. C. Hybridization as a source of variation for adaptation to new environments. Evolution 20, 315–336 (1966).
- Grant, P. R. & Grant, B. R. Phenotypic and genetic effects of hybridization in Darwin’s finches. Evolution 48, 297–316 (1994).
- Dannemann, M. & Kelso, J. The contribution of neanderthals to phenotypic variation in modern humans. Am. J. Hum. Genet. 101, 578–589 (2017).
- D’Angiolo, M. et al. A yeast living ancestor reveals the origin of genomic introgressions. Nature 587, 420–425 (2020).
- Bautista, C., Marsit, S. & Landry, C. R. Interspecific hybrids show a reduced adaptive potential under DNA damaging conditions. Evol. Appl. 14, 758–769 (2021).
- Carvajal, E., van den Hazel, H. B., Cybularz-Kolaczkowska, A., Balzi, E. & Goffeau, A. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol. Gen. Genet. 256, 406–415 (1997).
- Anderson, J. B., Sirjusingh, C. & Ricker, N. Haploidy, diploidy and evolution of antifungal drug resistance in Saccharomyces cerevisiae. Genetics 168, 1915–1923 (2004).
- Mutlu, N., Garipler, G., Akdoğan, E. & Dunn, C. D. Activation of the pleiotropic drug resistance pathway can promote mitochondrial DNA retention by fusion-defective mitochondria in Saccharomyces cerevisiae. G3 4, 1247–1258 (2014).
- Xia, H. et al. Evolutionary and reverse engineering in Saccharomyces cerevisiae reveals a Pdr1p mutation-dependent mechanism for 2-phenylethanol tolerance. Microb. Cell Fact. 21, 269 (2022).
- Taylor, M. B. et al. yEvo: experimental evolution in high school classrooms selects for novel mutations that impact clotrimazole resistance in Saccharomyces cerevisiae. G3 12, jkac246 (2022).
*Cover image created with the DALL·E tool by OpenAI.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in