Antibodies need teamwork!
Published in Chemistry, Microbiology, and Biomedical Research
Antibiotic resistance urgently calls for the development of alternative therapies against bacterial infections. A promising strategy is to boost the host immune system through antibodies, either indirectly via vaccination or directly via therapeutic antibodies. However, real progress in such developments is hampered by our limited knowledge of the processes underlying antibody-dependent immune clearance.
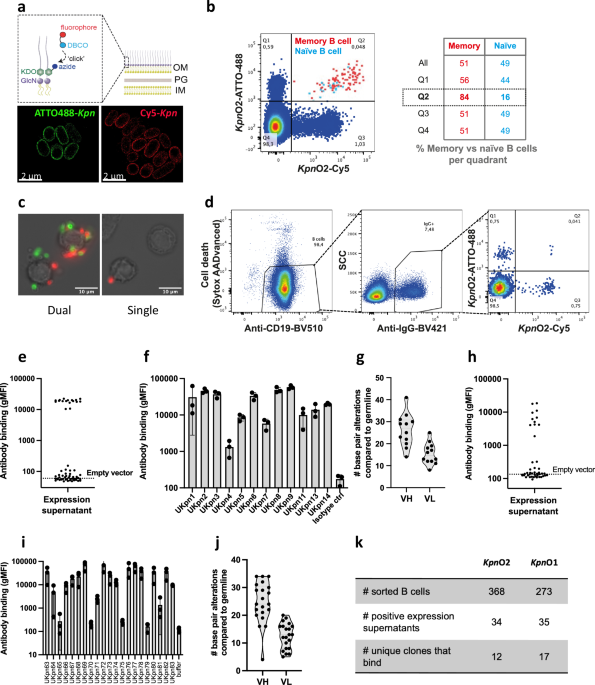
In this paper, we establish an approach to identify novel anti-bacterial antibodies from human B cells. Although it is common in the field to stain B cells with purified antigens, we decided to go for a more holistic approachand stain the B cells with entire living bacteria, thereby keeping antigens in the natural context of the complex bacterial membrane. Using this approach, we identified 29 novel antibodies against Klebsiella pneumoniae, an important cause of drug-resistant infections. Using genetic and functional approaches, we managed to couple the antibody’s functionality to its antigenic target.
1) The antigen matters!
2) Mixing helps!
We discovered that some antibodies can act synergistically. When added as a mixture, some antibodies can strengthen each other’s binding to the bacterial surface. Contrary to our expectation, this cooperation between antibodies was independent of the antibody’s Fc-tail.
In all, we anticipate that our approach will accelerate discovery of monoclonal antibodies against bacteria and stimulate others to explore whether antibody combinations are a promising route to develop potent antibody therapies.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in