Are your metabolic obesity phenotypes associated with the risk of overall and site-specific cancers?
Published in Cancer and General & Internal Medicine

Scientific rationale for the study
Modifiable lifestyle risk factors such as smoking, alcohol, unhealthy diet, obesity, and lack of physical activity, have been estimated to cause at least 40% of cancers, and about 4–8% of cancer cases are attributed to excess body weight. Although excess body fatness is a well-established risk factor for at least 13 different cancer sites, potential biological mechanisms underlying the adiposity–cancer relationship are not yet fully understood. Insulin resistance or hyperinsulinemia plays a more significant role in the obesity-cancer association; however, it is not clear whether the risk of obesity-related cancers differs amongst individuals with high adiposity but different metabolic health status. Previous studies have differed greatly in term of sample size, and results are conflicting and heterogeneous, mainly because the definition of metabolic health status varied across the studies. It is important to mention that metabolic obesity phenotypes are defined using a combination of adiposity and metabolic dysfunction parameters such as elevated levels of homeostasis model assessment of insulin resistance (HOMA-IR) index, fasting insulin, C-peptide, or having more than one metabolic abnormality.
What is our finding?
To improve our understanding and identify high-risk individuals among individuals with high body fatness, our recent meta-analyses of cohort studies published in the British Journal of Cancer is the largest meta-analysis on the association between metabolic obesity phenotypes and risk of cancer and included 31 publications (covering 15 unique cohort studies) published through June 2023 after screening a total of 15,556 records from several databases. Our article is available here.
We found that both metabolic dysfunction and excess body fatness increase the risk of multiple cancers, and the combination of adiposity and metabolic dysfunction is associated with the strongest increase in risk. Individuals classified as metabolically unhealthy overweight/obese were found to have a higher risk of several cancer types, including postmenopausal breast, colorectal, pancreatic, endometrial, gallbladder, stomach, bladder, liver, kidney, and thyroid cancer, compared to metabolically healthy normal weight individuals (Figure 1). In addition, we found elevated risks of most of these cancers among individuals classified as metabolically unhealthy normal weight or metabolically healthy overweight/obese compared to those classified as metabolically healthy normal weight phenotype. Our stratified analyses according to metabolic obesity phenotypes suggested that the elevated risks of some cancers were stronger in individuals classified as metabolically unhealthy overweight/obese versus those classified as metabolically healthy overweight/obese or metabolically unhealthy normal weight phenotypes.
Figure 1. Summary relative risks (SRRs) and 95% CIs for the association between metabolic obesity phenotypes and overall and site-specific cancer risk
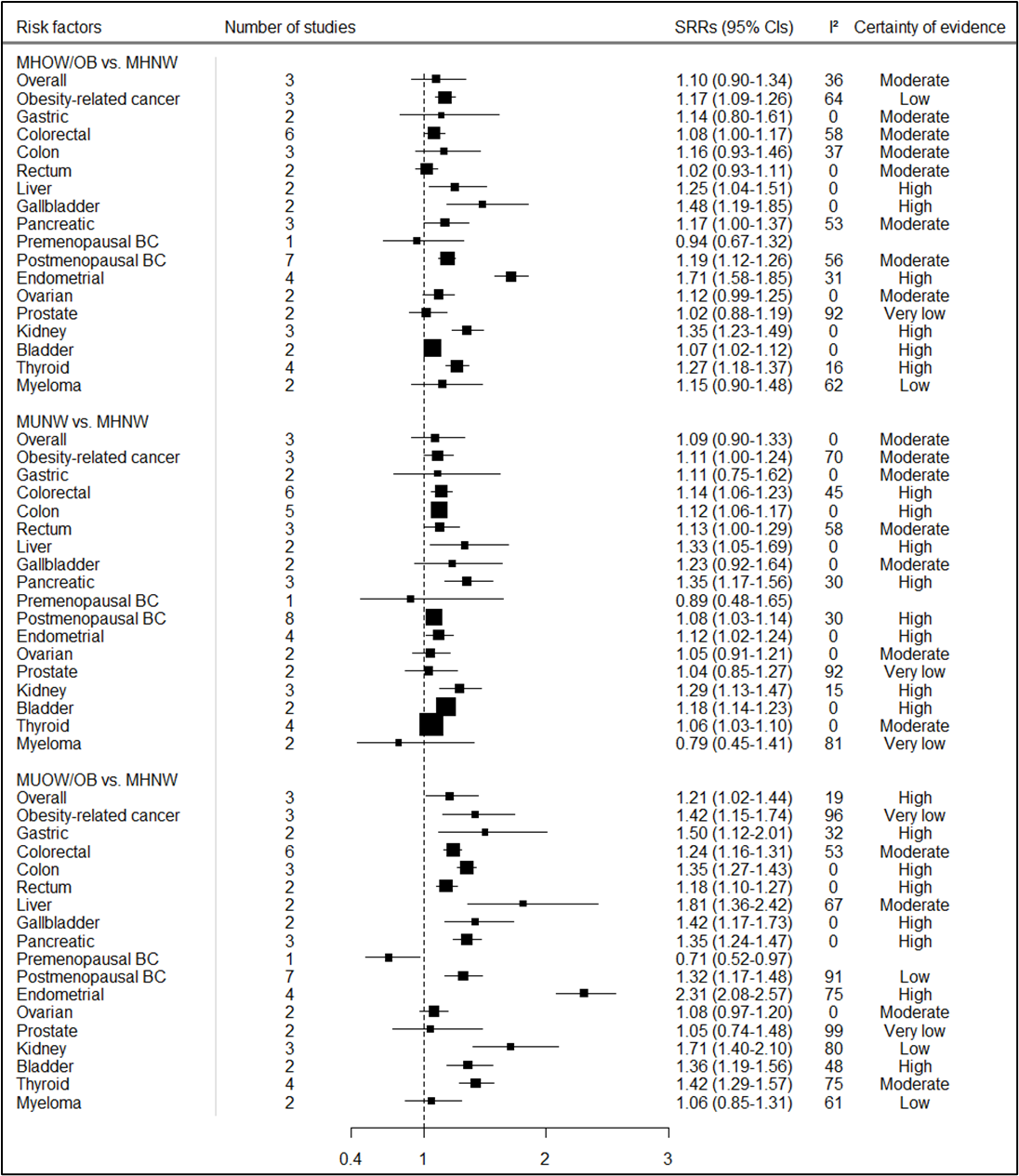
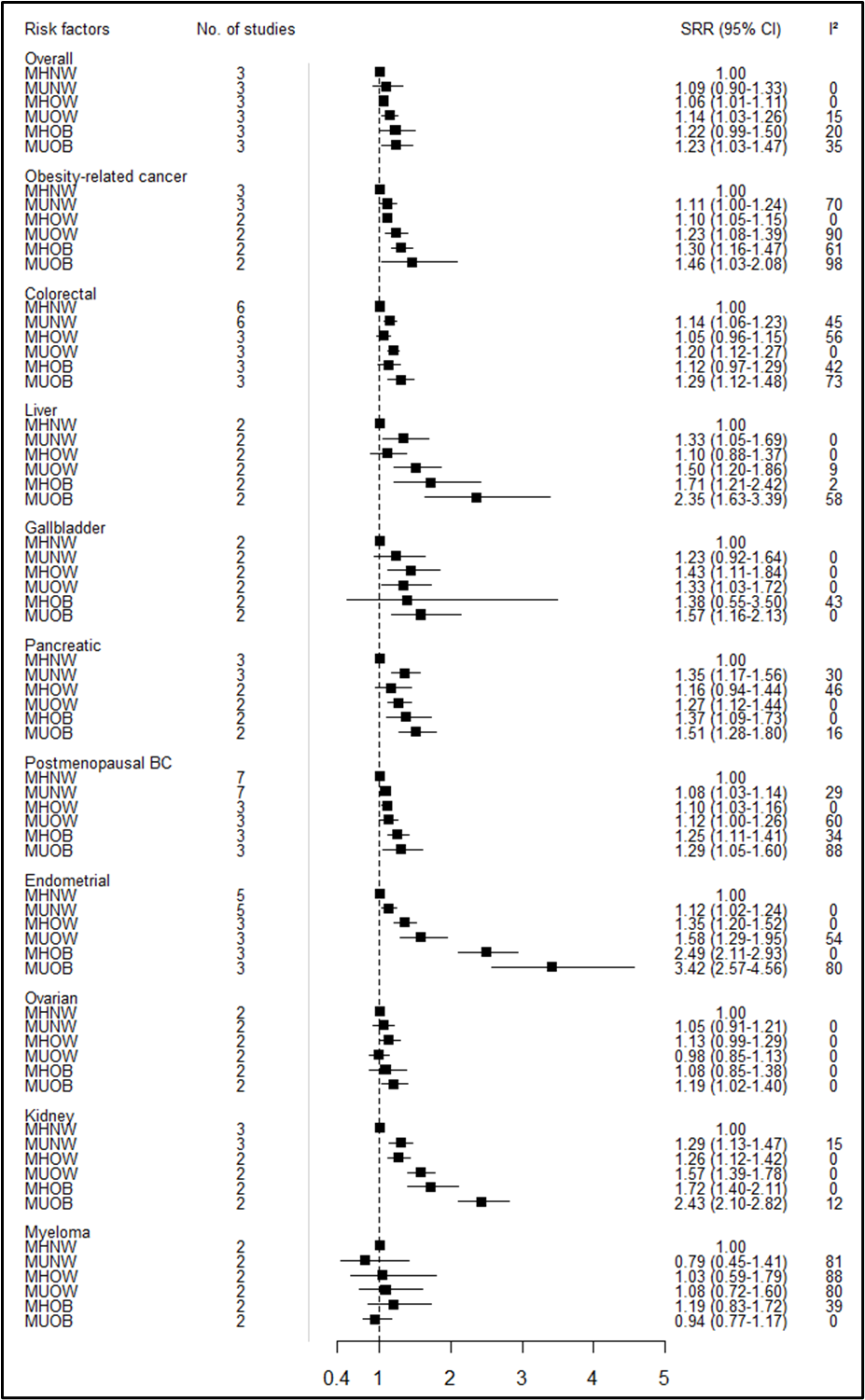
Subgroup analyses also suggest that there is a gradual increase in the risk for overall and obesity-related cancers, as well as some specific cancers such as postmenopausal breast, endometrial, and kidney cancer from metabolically unhealthy normal weight, through metabolically healthy overweight and metabolically unhealthy overweight to metabolically healthy obese and metabolically unhealthy obese by considering metabolically healthy normal weight as reference phenotype (Figure 2).
Figure 2. Summary relative risks (SRRs) and 95% CIs for the association between metabolic obesity phenotypes and overall and site-specific cancer risk separately for overweight and obesity
What does it tell us?
A possible mechanism underlying the elevated risk of cancer among metabolically unhealthy overweight/obese individuals may involve metabolic dysregulation, specifically insulin resistance, hyperinsulinemia, and diabetes. Insulin plays a crucial role in both normal and malignant cells, given that its receptor is commonly found in tumor cells; and insulin resistance can further promote cancer cell growth through its mitogenic and antiapoptotic activities. However, we also found that individuals classified as metabolically healthy overweight/obese had an elevated risk of obesity-related cancer, suggesting other mechanisms also may play a role. It is possible that chronic inflammation and sex steroid hormones resulting from excess adipose tissue may explain the higher risk of cancer. These findings suggest that the impact of adiposity on cancer incidence is likely to be at least partly independent of metabolic health.
To our knowledge, our meta-analysis is the first that assessed the risk of site-specific cancers in relation to metabolic obesity phenotypes. Notable strengths of this meta-analysis include its large sample size that ensured statistical power of findings, the detailed subgroup and sensitivity analyses, inclusion of different cancer subtypes and the cohort design of the included studies, reducing the potential for recall bias, selection bias, and reverse causation, which may affect case-control and cross-sectional studies. However, despite these strengths, several limitations of this review need to be considered such as the lack of information on changes in metabolic phenotype and adiposity over time and the fact that our findings did not take into account longitudinal changes in body weights or metabolic markers. Subgroup analyses stratified by use of medications including statins and metformin, duration of metabolic dysfunction, and by lifestyle such as physical activity, were not possible because of the lack of such data from the studies included.
Take-home message
In the context of the increasing prevalence of overweight and obesity which contributes to a large number of cancer cases, and since some individuals with overweight or obesity do not present any metabolic dysfunction, these findings may have important public health implications. Relying solely on anthropometric parameters to identify individuals at elevated risk of cancer could lead to the exclusion of normal-weight individuals with poor metabolic health; hence, this might underestimate the risk of cancer in individuals with poor metabolic health.
Although further cohort studies are needed to investigate changes in metabolic obesity phenotypes over time, these findings highlight the importance of combining measures of adiposity with indicators of metabolic dysfunction and this may be useful in identifying individuals at higher risk of cancer in addition to the existing screening practices.
This blog was written by Yahya Mahamat-saleh with comments from Dagfinn Aune.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in