Artificial intelligence unveils a golden (nano) secret
Published in Chemistry

Gold–what can it not do, and undo? - William Shakespeare
This question gets even more complex when looking at the phase stability of gold in the form of a nanoparticle, a system whose size ranges between 1 to 100 nms, corresponding to 1/100000th of the size of a human hair.
Gold nanoparticles' unique properties, which are exploited in catalysis, biomedicine, and optics, dramatically change depending on whether these are solid or liquid. Classical thermodynamics fails at the nano scale because of the non-trivial interplay between volume and surface effects towards the stabilization of a given phase. A mechanistic picture of the melting mechanism of small nanoparticles, as well as a tool to quantitatively predict their melting temperature, is key for their application in cutting-edge technologies.
To simulate what Gold nanoparticles do and don’t do when subject to heating, we resort to computational models which exploit quantum mechanics calculations, machine learning predictions, and molecular mechanics simulations. More specifically, we use machine learning to approximate the slow-but-accurate quantum mechanics predictions for forces acting among atoms. This way, atomic simulations that would take thousands of years if carried out using quantum mechanics first principles calculations, can be done in a few days, dramatically reducing their computational - and environmental - cost.
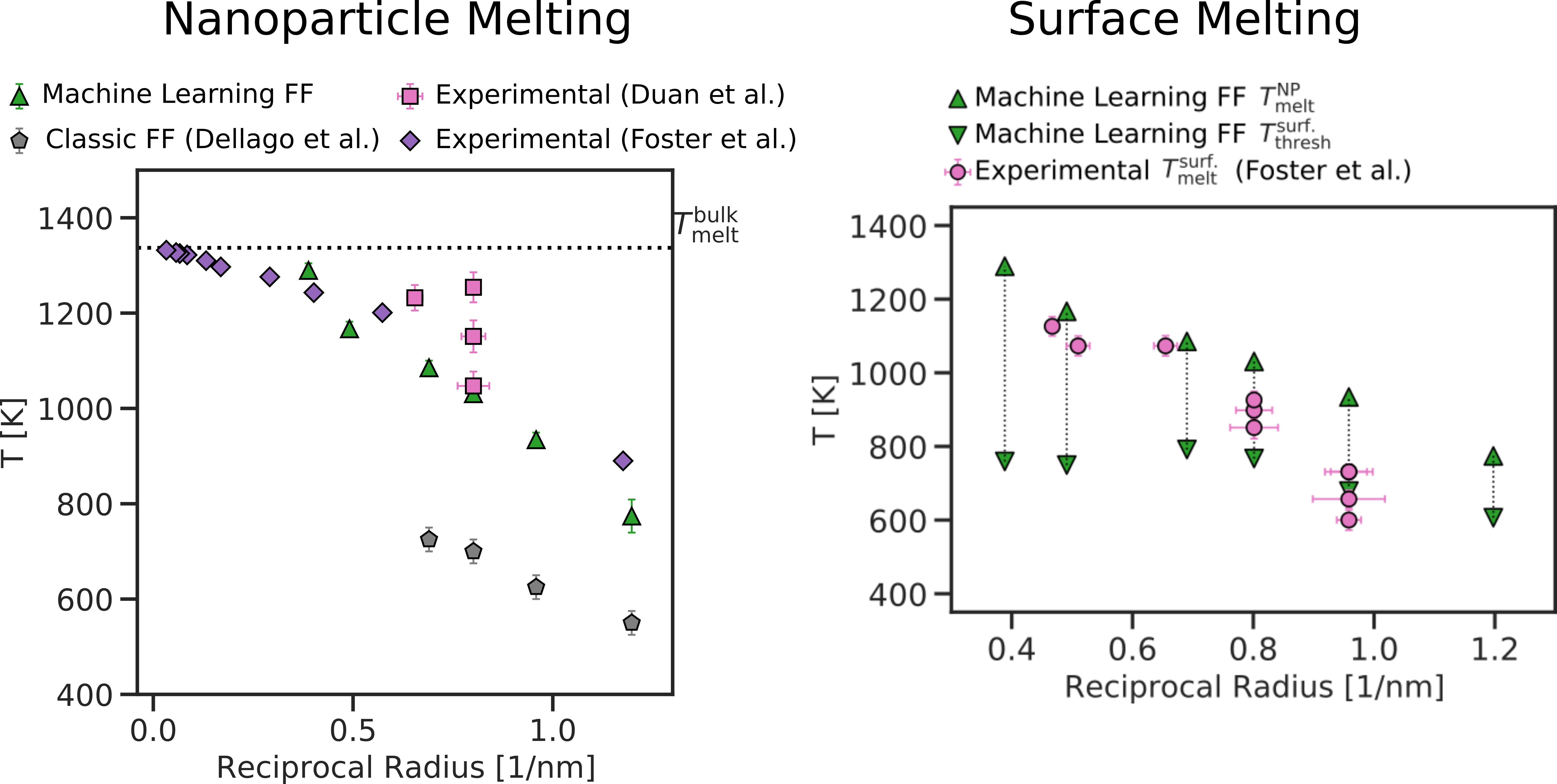
To analyse what happens inside the simulations we run, we apply unsupervised learning techniques to draw a data-driven criterion to define which ````"phase” each atom is in. Each atom is automatically identified as liquid or solid, and whether it belongs to the surface, the edge, or the inner part of the nanoparticle. This way, we can identify when and how the nanoparticles melt, and what happens at the surface at temperatures lower than the melting temperatures.
Our results, shown in Figure 1, predict the melting temperatures in good agreement with experiments, and highlight that atoms on the surface of nanoparticles become liquid earlier than the inner ones, at temperatures ranges that agree with experiments.
We foresee the application of the machine learning tools here presented towards addressing the complexity of phase changes in other technologically-relevant systems.
The paper:
Claudio Zeni, Kevin Rossi, Theodore Pavloudis, Joseph Kioseoglou, Stefano de Gironcoli, Richard E. Palmer & Francesca Baletto Data-driven simulation and characterisation of gold nanoparticle melting Nature Communications volume 12, Article number: 6056 (2021)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in