Arylcarboxylation of unactivated alkenes with CO2 via visible-light photoredox catalysis
Published in Chemistry
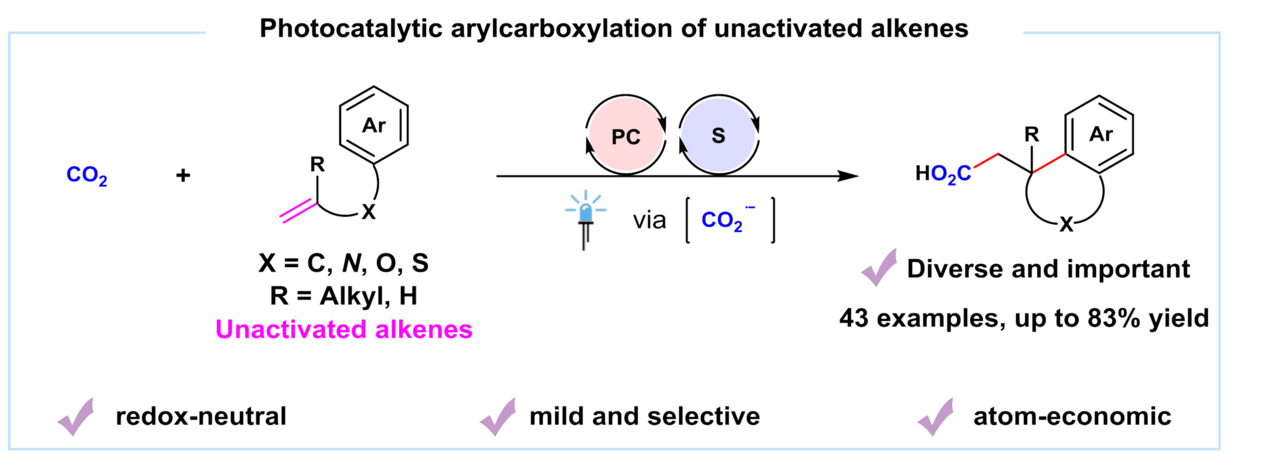
The research group of Prof. Da-Gang Yu and Prof. Jian-Heng Ye at Sichuan University focuses on the development of strategies for the activation and transformation of CO2 via visible-light photoredox catalysis in the past few years (key review: Acc. Chem. Res. 2021, 54, 2518). We have developed the visible-light-driven hydro-and difunctionalization of activated alkenes (ACIE 2017, 56, 15416; Nat. Commun. 2019, 10, 3592; CCS Chem.2020, 2, 1746; Sci. China Chem. 2021, 64, 1164; JACS 2021, 143, 2812; Nat. Catal. 2021, 4, 304), umpolung carboxylation of enamides/imines/carbonyl compounds (ACIE 2018, 57, 13897; Nat. Commun. 2021, 12, 3306), remote difunctionalizing carboxylation of unactivated alkenes (ACIE 2020, 59, 21121) and hydro-and di-carboxylation of unactivated alkenes via ConPET (Nat. Catal. 2022, 5, 832). In our recent paper published on Nature Communications, we described the latest progress on the topic of photocatalytic carboxylation. A synergistic merger of photoredox and hydrogen atom transfer catalysis enables arylcarboxylation of unactivated alkenes with CO2, affording a variety of valuable polycyclic carboxylic acids and derivatives, which are widely found in natural products, drugs and bioactive compounds, as shown below.
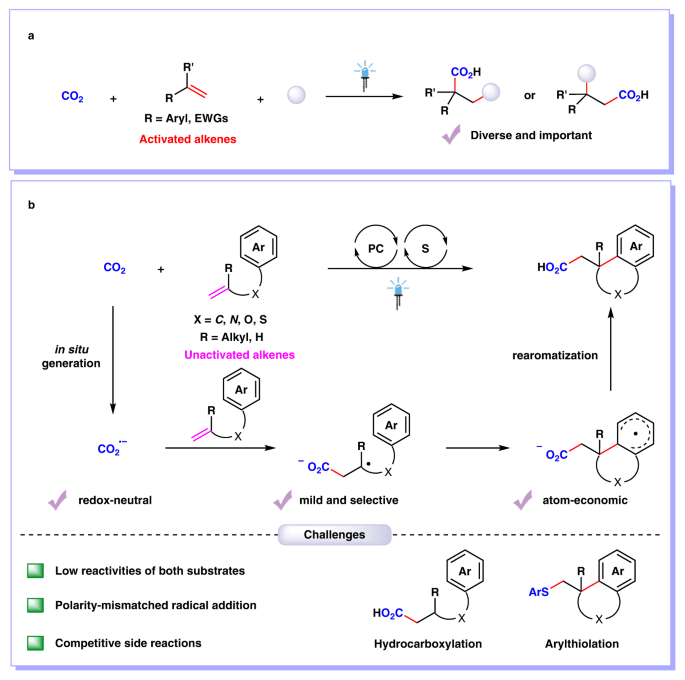
Photocatalytic difunctionalization of alkenes with CO2 represents a powerful strategy for accessing high value-added carboxylic acids with increased complexity and diverse functionality1,2. In recent years, several examples of visible-light photoredox-catalyzed 1,2-difunctionalizing carboxylation of alkenes have been reported by Martin3, Wu4, Li5, Xi6 and our group7-10, independently. However, most methods are mainly limited to activated alkenes (e.g., aryl alkenes or acrylates). In contrast, the unactivated alkenes, which are more easily available in nature and industry but are more challenging substrates as well, have been rarely investigated in visible-light photocatalytic carboxylation with CO2 due to the low reactivity of both substrates11. Moreover, it is difficult to reduce either unactivated alkenes or CO2 to their radical anions, which might act as key intermediates in such carboxylation. Besides, the addition of nucleophilic CO2•− to electron-rich unactivated alkenes is a polarity-mismatched process. In addition, hydrocarboxylation, arylthiolation and other competitive side reactions would also hamper the desired arylcarboxylation reaction.
With these challenges in mind, we envisioned and successfully realized the arylcarboxylation of unactivated alkenes with CO2 via the synergistic merger of photoredox and hydrogen atom transfer catalysis. The desired reaction takes place in an efficient and sustainable manner, showing high chemo- and regio-selectivity, broad substrate scope, good functional group tolerance as well as mild conditions, delivering a wide variety of valuable polycyclic acids and derivatives in moderate-to-good yields. Mechanistic investigations indicate that in situ generated CO2•− might act as a key intermediate involved in this transformation.
More details of this work could be found here: “Arylcarboxylation of unactivated alkenes with CO2 via visible-light photoredox catalysis” in Nature Communications.
Reference
- Zhang, Z. et al. Radical-Type Difunctionalization of Alkenes with CO2. Acta Chim. Sinica 77, 783 (2019).
- Bertuzzi, G., Cerveri, A., Lombardi, L. & Bandini, M. Tandem functionalization-carboxylation reactions of π-systems with CO2. J. Chem. 39, 3116-3126 (2021).
- Yatham, V. R., Shen, Y. & Martin, R. Catalytic intermolecular dicarbofunctionalization of styrenes with CO2 and radical precursors. Chem. Int. Ed. 56, 10915-10919 (2017).
- Hou, J. et al. Visible-light-mediated metal-free difunctionalization of alkenes with CO2 and silanes or C(sp3)-H alkanes. Chem. Int. Ed. 57, 17220-17224 (2018).
- Wang, H., Gao, Y., Zhou, C. & Li, G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. Am. Chem. Soc. 142, 8122-8129 (2020).
- Zhang, B., Yi, Y., Wu, Z.-Q., Chen, C. & Xi, C.-J. Photoredox-catalyzed dicarbofunctionalization of styrenes with amines and CO2: a convenient access to γ-amino acids. Green Chem. 22, 5961-5965 (2020).
- Ye, J.-H. et al. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Chem. Int. Ed. 56, 15416-15420 (2017).
- Fu, Q. et al. Transition metal-free phosphonocarboxylation of alkenes with carbon dioxide via visible-light photoredox catalysis. Nat. Commun. 10, 3592 (2019).
- Liao, L.-L. et al. α-Amino Acids and Peptides as Bifunctional Reagents: Carbocarboxylation of Activated Alkenes via Recycling CO2. Am. Chem. Soc. 143, 2812-2821 (2021).
- Ju, T. et al. Dicarboxylation of alkenes, allenes, and (hetero)arenes with CO2 via visible-light photoredox catalysis. Catal. 4, 304-311 (2021).
- Song, L. et al. Visible-Light Photocatalytic Di-and Hydro-Carboxylation of Unactivated Alkenes with CO2. Catal. 5, 832-838 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in