Asymmetric dearomatization catalysed by chiral Brønsted acids via activation of ynamides
Published in Chemistry
Our group has been focusing on ynamide chemistry for synthesizing various valuable N-containing heterocycles. Based on different strategies such as oxidation, amination and cycloisomerization, we realized a series of novel transformations of ynamides via catalysis of metals such as Au, Pt, Cu, Zn, Y etc.[1] Furthermore, we considered the possibility of exploring a new catalytic mode by employing Brønsted acids instead of those transition metals to activate ynamides. Although some pioneering work of our group in this area have been published,[2−4] a number of challenges are still remained, especially the asymmetric synthesis with high efficiency and enantioselectivity.
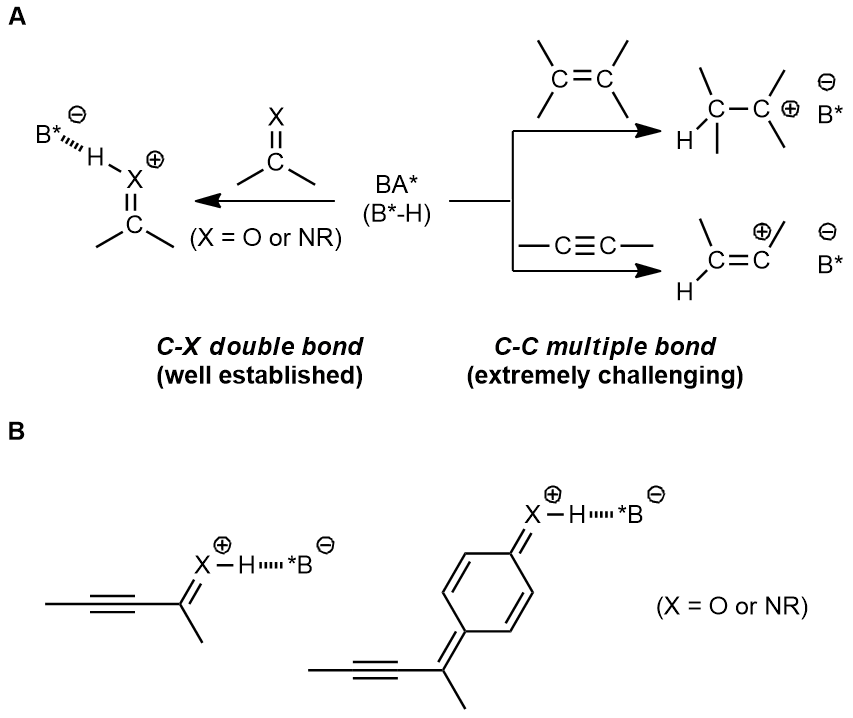
Chiral Brønsted acids have been widely employed in asymmetric synthesis over the past decades, and numerous efficient synthetic methods have been developed based on this approach. However, those transformations were always triggered by the direct activation of carbon-heteroatom double bonds, such as imine and carbonyl compounds. It is explicit that the protonation of imines and carbonyls by chiral Brønsted acid leads to a stable intermediate, hydrogen-bonding with the conjugate base of chiral Brønsted acid. The hydrogen bond is considered as an anchor to generate a rigid structure of the transition state, which is beneficial for stereocontrol. In contrast, protonation of alkenes and alkynes affords a carbocation species, connecting with conjugate base of chiral Brønsted acid through a relatively weak electrostatic interaction, which is unfavorable in controlling the enantioselectivity (Fig. A). Although the direct activation of different kinds of C-C double bonds such as dienes[5], electron-rich alkenes[6] and unbiased olefins[7] through chiral Brønsted acid have been reported in a few examples, the direct activation of alkynes has so far not been invoked. Of note, electron-efficient alkynes could be involved in chiral Brønsted acid catalysis, where chiral Brønsted acid were essentially used to activate the imine and carbonyl moieties (Fig. B) but not carbon–carbon triple bonds.
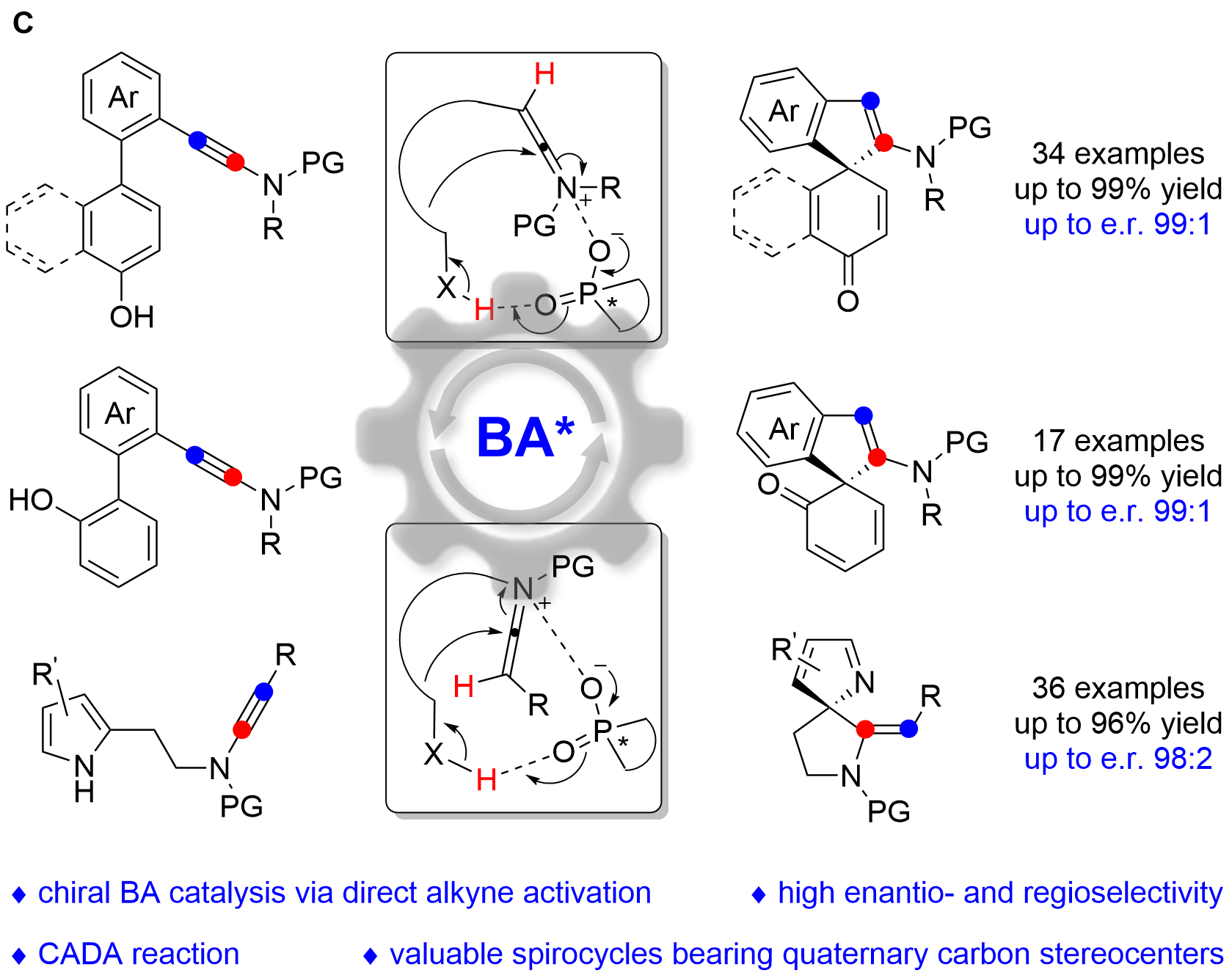
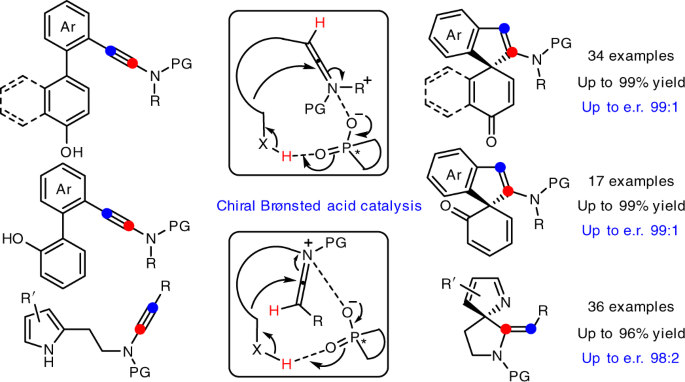
We herein report that chiral Brønsted acid enable the catalytic asymmetric dearomatization (CADA) reactions of naphthol-, phenol- and pyrrole-ynamides by direct activation of ynamides, which represents the first chiral Brønsted acid catalysis via the direct activation of carbon–carbon triple bonds (Fig. C). At the beginning, naphthol-ynamide substrates were designed and employed for the optimization of CADA reaction. Gratifyingly, with the chiral Brønsted acid bearing a skeleton of BINAP, we could realized the practical and atom-economical synthesis of a wide range of valuable spirocyclic enones with a chiral quaternary carbon stereocenter in generally high yields with excellent enantioselectivities (up to 99:1 e.r.). Furthermore, we subsequently synthesized the ortho-substituted phenol-ynamides, which are more challenging to be dearomatized because of the relatively stronger aromaticity of phenols. To our delight, through the extensive optimization, the corresponding spirocyclic products could be obtained with satisfying results (up to 99:1 e.r.) and the seven-membered byproduct led by O-cyclization could be perfectly inhibited. What’s more, the substrate scope could be further extended to pyrrole-ynamides, furnishing valuable chiral spirocyclic 2H-pyrroles in good to excellent yields with high enantioselectivities (up to 98:2 e.r.). Significantly, excellent E/Z ratios (>20:1) were detected in these cases.
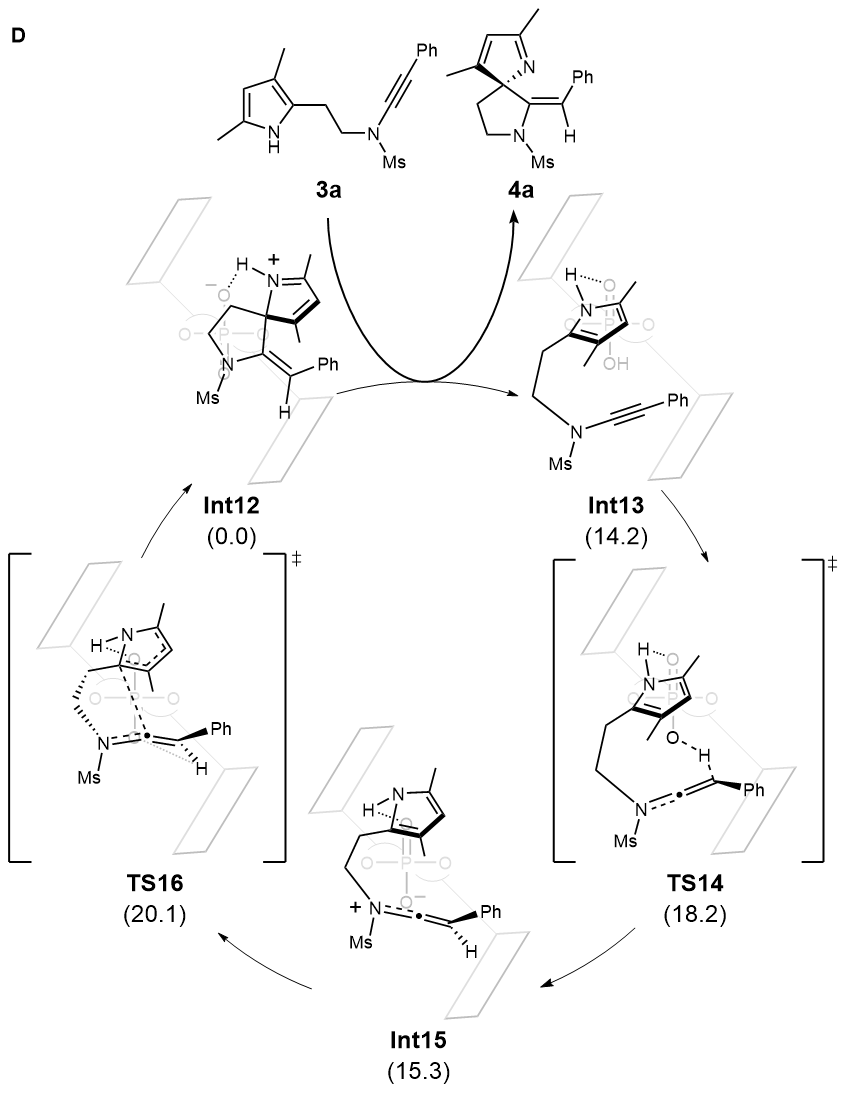
Density functional theory (DFT) calculations based on pyrrole-ynamide substrate were also carried out. The results indicated that a rapid and reversible protonation process of substrate leads to a keteniminium intermediate, attaching to chiral catalyst with both hydrogen bonding and ion pair interactions. The further nucleophilic attack of pyrrole ring with the irreversible formation of C-C bond was identified as the rate- and enantioselectivity-determining step, affording the corresponding product and releasing the catalyst (Fig. D).
We hope this chiral Brønsted acid-catalysed asymmetric dearomatization reaction could open a new horizon of ynamide chemistry and asymmetric synthesis. And this novel activation mode of chiral Brønsted acid catalysis is expected to be of broad utility in catalytic asymmetric reactions involving ynamides and the related heteroatom-substituted alkynes
References:
- Hong, F.-L. & Ye, L.-W. Transition-metal catalyzed tandem reactions of ynamides for divergent N-heterocycle synthesis. Chem. Res. 53, 2003−2019 (2020).
- Xu, Y. et al. Organocatalytic enantioselective Conia-ene-type carbocyclization of ynamide-cyclohexanones: regiodivergent synthesis of morphans and normorphans. Chem. Int. Ed. 58, 16252−16259 (2019).
- Zhou, B. et al. Stereoselective synthesis of medium lactams enabled by metal-free hydroalkoxylation/stereospecific [1,3]-rearrangement. Commun. 10, 3234 (2019).
- Li, L. et al. Metal-free alkene carbooxygenation following tandem intramolecular alkoxylation/Claisen rearrangement: stereocontrolled access to bridged [4.2.1] lactones. Sci. 10, 3123−3129 (2019).
- Shapiro, N. D., Rauniyar, V., Hamilton, G. L., Wu, J. & Toste, F. D. Asymmetric additions to dienes catalysed by a dithiophosphoric acid. Nature 470, 245−249 (2011).
- Čorić, I. & List, B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature 483, 315−319 (2012).
- Tsuji, N. et al. Activation of olefins via asymmetric Brønsted acid catalysis. Science 359, 1501−1505 (2018).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in