Autism’s Many Faces: Why Studying Different Models Matters
Published in Neuroscience and General & Internal Medicine

When we first started researching autism spectrum disorder (ASD), one question kept coming up in our mind: Why does ASD look so different from one individual to another? Even though the famous “autism triad” is always present – altered communication, reduced socialization and repetitive behaviours - it is widely recognized that some people predominantly experience social communication challenges, others repetitive behaviors, and many present unique combinations of both. Besides, symptoms such as altered sensory processing or difficulty in motor coordination are also highly reported in individuals with ASD, at differing degrees of severity. These differences are even reflected in how individuals respond to therapies. This heterogeneity is one of the biggest challenges in ASD research, and a fundamental reason why studying different animal models of ASD is so critical.
ASD is not one single condition but a spectrum, and genetics plays a big part in its diversity. For example, syndromes like neurofibromatosis type 1 (NF1) and tuberous sclerosis complex type 2 (TSC2) are both associated with a higher likelihood of ASD, but they affect different biological pathways. Indeed, while NF1 is characterized by reduced excitability, TSC2 shows an increase in the E/I ratio of neuronal excitation. So, we wanted to know: do these genetic differences translate into differences in early development?
There is a sex gap in autism
For decades, ASD was thought to be 4 times more common in boys than in girls. More recent studies, however, have revised this ratio to about 3 boys for every girl. This change reflects the important realization that many girls and women with ASD were underdiagnosed throughout the history of this condition. Subtler or different symptom profiles in girls, as well as cultural expectations of female behavior, often meant they were overlooked or misdiagnosed, resulting in a delayed recognition of the problem and a later access to support and treatment.
Despite this knowledge, many animal studies still focus exclusively on male subjects, discarding females altogether, blaming it on the “female estrous variability” (which is also very debatable) to justify not studying them. This is a critical limitation because males and females can differ in their symptoms, developmental trajectories, and even their responses to therapies. Understanding these sex-driven differences is essential, not only to capture the full spectrum of ASD but also to pave the way for more inclusive and effective treatment strategies.
In our group, we are deeply committed to investigating both males and females in neurodevelopment and unraveling the role of biological sex in the onset and progression of diseases and conditions. As such, it was clear that in this study, we had to analyze both sexes of NF1 and TSC2 mouse pups.
Looking at the very beginning
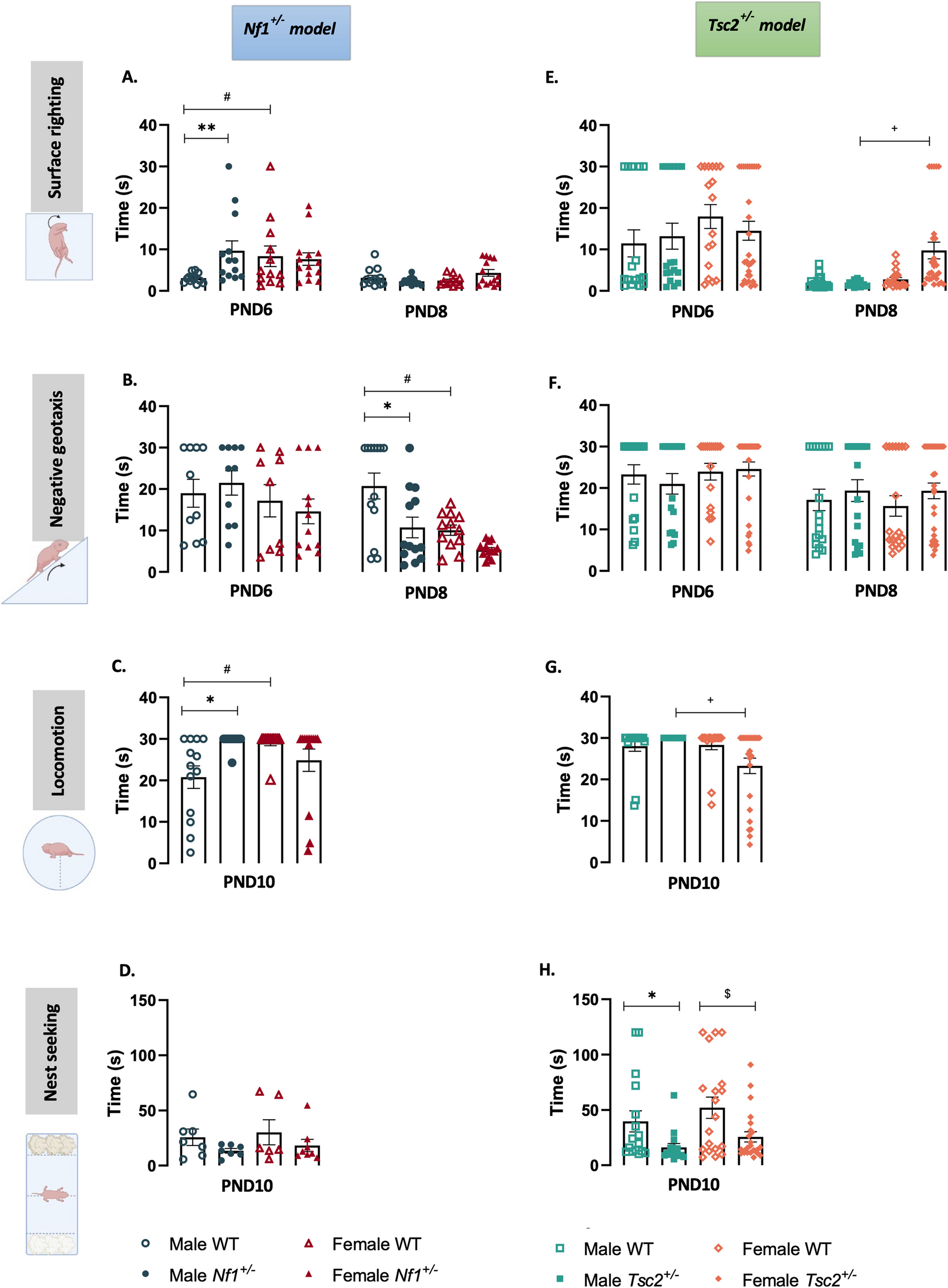
Although ASD is diagnosed in childhood, surprisingly few animal studies examine neonatal development, which corresponds to the period when many early biological differences are likely to appear. We decided to focus on NF1 and TSC2 mice, as these are two animal models of ASD with opposing molecular imbalances, and investigated developmental milestones, namely motor skills and coordination, and maternal separation-induced ultrasonic vocalizations, an early window into communication abilities. Importantly, we performed a longitudinal study across three developmental timepoints, at postnatal days 6, 8 and 10. This is something not so often performed in the field, with the majority of studies preferring to focus on one specific timepoint. However, this was essential to capture how developmental curves unfolded over time.
Tiny Pups, Big Responsibility
This work was performed in mouse pups at an incredibly early age: from postnatal day 6 until postnatal day 10. Working with newborn mouse pups was one of the most delicate aspects of this project. These pups are tiny, fragile, and completely dependent on their mothers, which meant every experiment and handling had to be performed with extreme care, skill and patience, as even small mistakes could have big consequences for their well-being.
We spent a great deal of preparation time refining the experimental protocol to be able to assess their developmental milestones and ultrasonic vocalizations without causing unnecessary stress. For instance, the mothers of each litter were previously habituated to us, the experimental operators, to make sure that maternal stress, which has a great impact on the offspring’s behavior, was kept to a minimum; on the day of each test, the litters were calmly transported to the experimental room and let acclimatize for a generous amount of time, again to reduce stress; and even on non-experiment days, husbandry of the animals was always performed by the same experimental operators, to avoid the stress triggered by the contact with an unfamiliar person. It was both a challenge and a responsibility: knowing that these early-life experiments could reveal subtle differences critical for understanding ASD made us even more committed to doing it right.
Two syndromes, two developmental curves
What we found was striking: Nf1+/- and Tsc2+/- pups showed very different developmental profiles. In Nf1+/- pups, we observed clear genotype-driven differences early on, while Tsc2+/- pups showed mainly strong sex-dependent differences instead. Even the ultrasonic vocalizations, namely their number, frequency modulation, or call complexity, were distinct. In other words, these two genetic models of ASD start life on very different developmental paths, which translates to distinct motor, coordination and communication skills at each timepoint.
Why this matter
These differences are not just academic details. They reflect how diverse ASD really is. Not only are individuals in themselves unique, but the biological syndromes associated with ASD produce their characteristic profiles. The story of an individual with ASD is not defined at diagnosis. This may help explain why one-size-fits-all treatments often fail and why personalized strategies are crucial. Studying heterogeneity early in life may help us better understand the roots of ASD and guide more tailored therapeutic approaches. This is an essential step towards improving the quality of life and independence of the people living with ASD, and of their friends and families.
Follow the Topic
-
Journal of Neurodevelopmental Disorders

This is an open access journal that integrates current, cutting-edge research across a number of disciplines, including neurobiology, genetics, cognitive neuroscience, psychiatry and psychology.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in