Understanding How Sex-Specific Brain Networks Influence Social Deficits in Autism
Published in Neuroscience
Why do boys and girls show differences in autism symptoms?
Autism Spectrum Disorder (ASD) is more common in boys than in girls, but we still don’t fully understand why. One possibility is that the brains of males and females are wired differently, and that these differences affect how ASD-related symptoms emerge and evolve.
In our recent study published in Translational Psychiatry, we investigated this question using a powerful mouse model of ASD. What we found was striking: male and female mice with the same genetic mutation showed similar behavioral problems, but the brain networks behind those problems were completely different. This discovery could help explain why autism looks different in boys and girls, and it offers new insights into the neural circuits involved in social behavior.
Our research question: Same behavior, different brain?
Scientists often use mouse models to understand the biological basis of autism. In this case, we used a well-known genetic model: mice that lack a gene called Tsc2, which is strongly linked to ASD in humans. These mice show core autism-like symptoms, especially social deficits.
We wanted to ask: are the brain circuits involved in these social deficits the same in male and female mice?
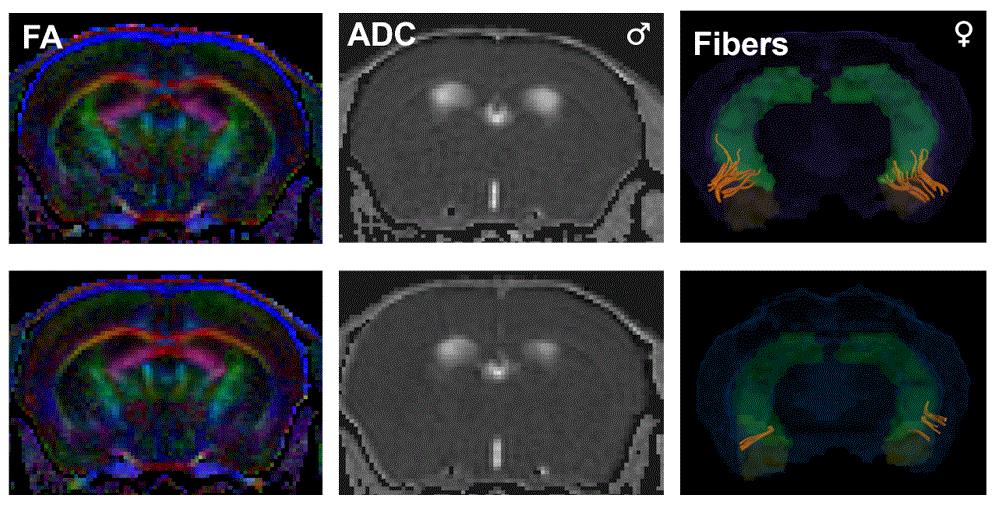
To find out, we combined behavioral tests and neuroimaging tools. This lets us map functional networks across the cortex, essentially, how different regions of the brain "talk" to each other during social behaviors.
What we found: Sex-specific brain signatures
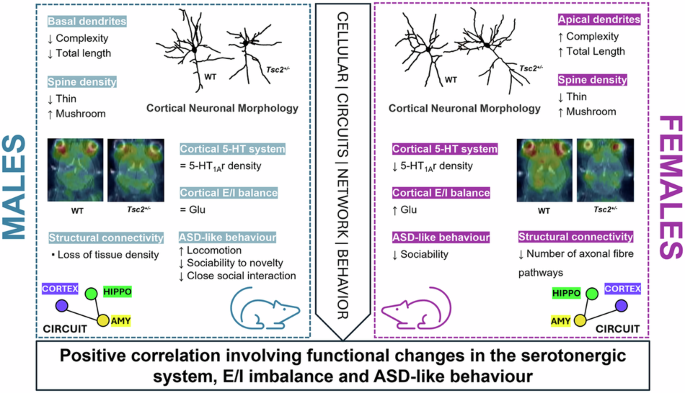
Even though both male and female mutant mice showed similar behavioral impairments (like reduced interest in social interaction), the underlying brain networks were quite different.
This sex-specific brain mapping suggests that males and females may “arrive” at the same social symptoms through different neural pathways — a concept known as "different roads to the same destination."
Why does this matter?
This finding is important for a few reasons:
- It supports the idea of sex differences in the neurobiology of autism. While previous studies have shown that autism may affect males and females differently, our work shows these differences at the level of functional brain networks.
- It opens the door for personalized therapies. If the brain mechanisms of social deficits are different in males and females, then treatments (whether behavioral or pharmacological may need to be tailored accordingly.
- It helps refine our models of ASD. Many studies lump together male and female subjects, assuming the results are interchangeable. Our data suggest this approach may miss critical sex-specific patterns.
What comes next?
We're now working to understand what causes these sex-specific patterns. Are hormones involved? Are these networks shaped differently during development? Could early interventions rewire them?
We also want to explore how these circuits relate to other features of autism, such as sensory processing or anxiety, and whether similar patterns exist in human brain imaging data from boys and girls with ASD.
Final thoughts: Embracing complexity
Autism is a complex condition with many causes and manifestations. Our study adds to the growing recognition that sex is a key factor in this complexity, not just in how autism appears, but in how it’s wired into the brain.
By embracing that complexity and using advanced tools to dissect it, we can move toward a deeper understanding of ASD and, ultimately, more effective and inclusive ways to support all individuals on the spectrum.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Jun 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in