AvmM Catalyses Macrocyclization through Dehydration/Michael-type Addition in Alchivemycin A Biosynthesis
Published in Chemistry

The biosynthesis of various types of natural products such as polyketides, non-ribosomal peptides and terpenoids is based on the core enzymes polyketide synthase (PKS), non-ribosomal peptide synthase (NRPS), and terpene synthase (TS). The core enzyme takes the primary metabolic molecule as the basic structural unit and performs specific directional condensation to generate the complex initial skeleton of natural products. Subsequently, post-modification enzymes can further carry out various specific modifications to the backbone molecules, ultimately resulting in complex natural products with diverse structures and activities. The catalysis of these post-modifying enzymes not only imparts structural complexity and diversity to natural products, but is often closely related to the biological activities or functions of the compounds. Therefore, the study of post-modifying enzymes of natural products is crucial for our understanding of the diverse biosynthetic processes of natural products and the discovery of new catalytic mechanisms.
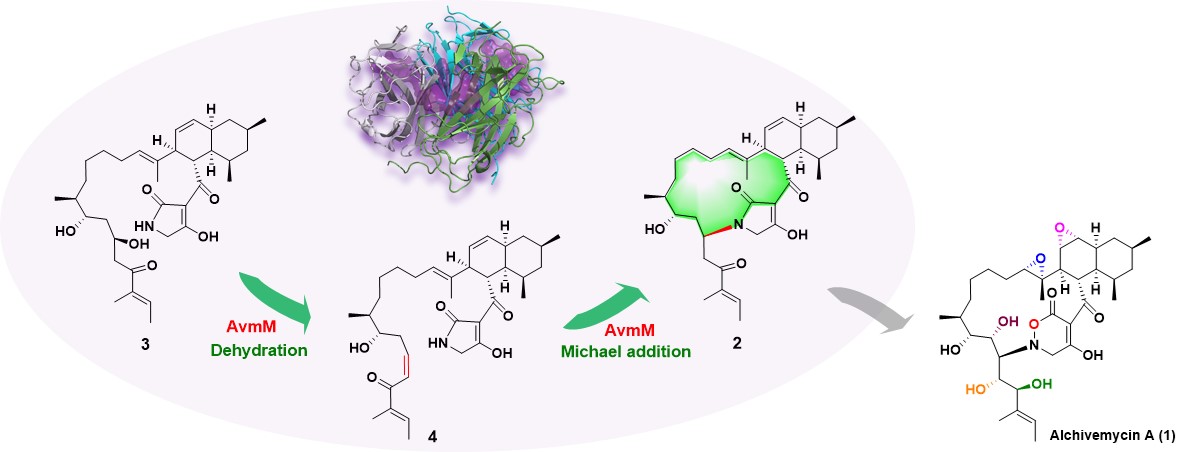
Fig. 1 AvmM catalyzes macrocycle formation in alchivemycin A biosynthesis.
Alchivemycin A (AVM, 1) is a potent antibacterial polycyclic polyketide with an unprecedented skeleton originally isolated from a plant-derived actinomycete Streptomyces sp. TP-A0867. AVM contains a 17-membered macrocyclic ring, featuring a cis-decalin, a rare 2H-tetrahydro-4,6-dioxo-1,2-oxazine (TDO) moiety, and two epoxide rings. AVM is biosynthetically derived from a hybrid cis-AT polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) pathway. In a previous study, we elucidated six consecutive redox steps in AVM biosynthesis mediating the formation of one TDO ring, two epoxy rings, and three hydroxyl groups. But the formation process of its macrocyclic skeleton is still unknown and fascinating.
Macrocyclization from linear precursors to cyclized products can afford constrained three-dimensional structures, which are important for their proper biofunctionality. The thioesterase (TE)-mediated macrocyclization is regarded as the most canonical strategy in polyketide and nonribosomal peptide biosynthesis, where a hydroxyl or an amino group in the polyketide or peptide acyl chain acts as an internal nucleophile to attack the TE-tethered carbonyl carbon, generating a macrolactone or a macrolactam. Another effective way to form macrocycles is oxidative coupling catalyzed by P450, rieske oxygenase, or radical S-adenosyl-L-methionine (SAM) enzymes, as exemplified by the biosynthesis of vancomycin, metacycloprodigiosin and streptide. In addition, nature adapts a number of noncanonical enzymatic machineries to form diverse macrocycles. For instance: a [4+2] Diels-Alderase, PyrI4, is responsible for the macrocyclization of the aglycone of pyrroindomycin and its structurally-related compounds including versipelostatin, kijanimicin, and tetrocarcin; a dual functional enzyme, LkcE, catalyzes a unique amide oxidation and a subsequent Mannich reaction to form the polyketide macrocycle in the lankacidin biosynthesis. Therefore, understanding how nature generates macrocyclic molecules from acyclic precursors may provide new inspiration for the development of bio/synthetic methodologies。
Recently, our detailed elucidation of the construction of the 16-membered ring in AVM biosynthesis was published in Nature Communications (available here). We first confirmed that the formation of the 16-membered ring was related to avmM by gene knockout, and the mutant ΔavmM accumulated the uncyclized linear intermediate 3, and in vitro enzyme catalysis experiments also proved that AvmM can indeed catalyze the cyclization of 3 to form compound 2. According to the labeling experiments, we speculate that AvmM catalyzes tandem β-elimination and Michael addition to form a C(sp3)-N bond, thereby constructing the 16-membered N-heterocycle of AVM. Further bioinformatics analysis found that AvmM is a new protein with no sequence similarity in the database, so we crystallized the crystal structures of AvmM and AvmM-2, and combined point mutation experiments, density functional theory (DFT) calculations and molecular dynamics (MD) simulations to speculate its specific catalytic mechanism.
Although a large number of natural products with macrocyclic structures have been reported, most of them are mediated by the TE. After the release of the PKS or NRPS chains, the formation of macrocycles catalyzed by other post-modifying enzymes is relatively infrequent. And our study found that AvmM catalyzes 16-membered ring formation efficiently in a way that has never been reported before, which may be promising in the development of biocatalytic tools.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in