Barnacle-glue-inspired paste for rapid and coagulation-independent hemostatic sealing
Published in Bioengineering & Biotechnology

Stop Bleeding - Lingering Clinical Challenge
The human body is full of densely packed large and small blood vessels from the heart to our fingertips, making our body almost like a blood sponge. As a result, bleeding - an event of leaking blood from damaged/injured tissues and organs - is an inevitable part of all surgeries and injuries. Excessive loss of blood by bleeding can cause substantial clinical and economic burdens from the increased cost of treatment to multiple times higher rate of mortality. Hence, stopping bleeding or hemostasis is one of the most critical requirements in the treatment of patients both in operating rooms for surgeries and in fields for traumatic/emergency injuries.

Severe and anti-coagulated bleeding in ex vivo porcine aorta
Despite being a routine and critical problem, hemostasis is a lingering clinical challenge. Failure to stop bleeding and associated complications is still the 1st cause of mortality in the military and 2nd cause of death in the civilian sectors in the U.S. (before the COVID-19 pandemic), costing more than 1 M live every year globally. While numerous conventional techniques and commercially available hemostatic agents have been developed and used, they are far from solving the challenge. The challenge is the starkest for bleedings where hemostasis is most needed - patients with severe or hard-to-stop (for example, anti-coagulated or coagulopathic) bleedings. There are no available solutions that can rapidly and effectively stop severe or anti-coagulated/coagulopathic bleedings.
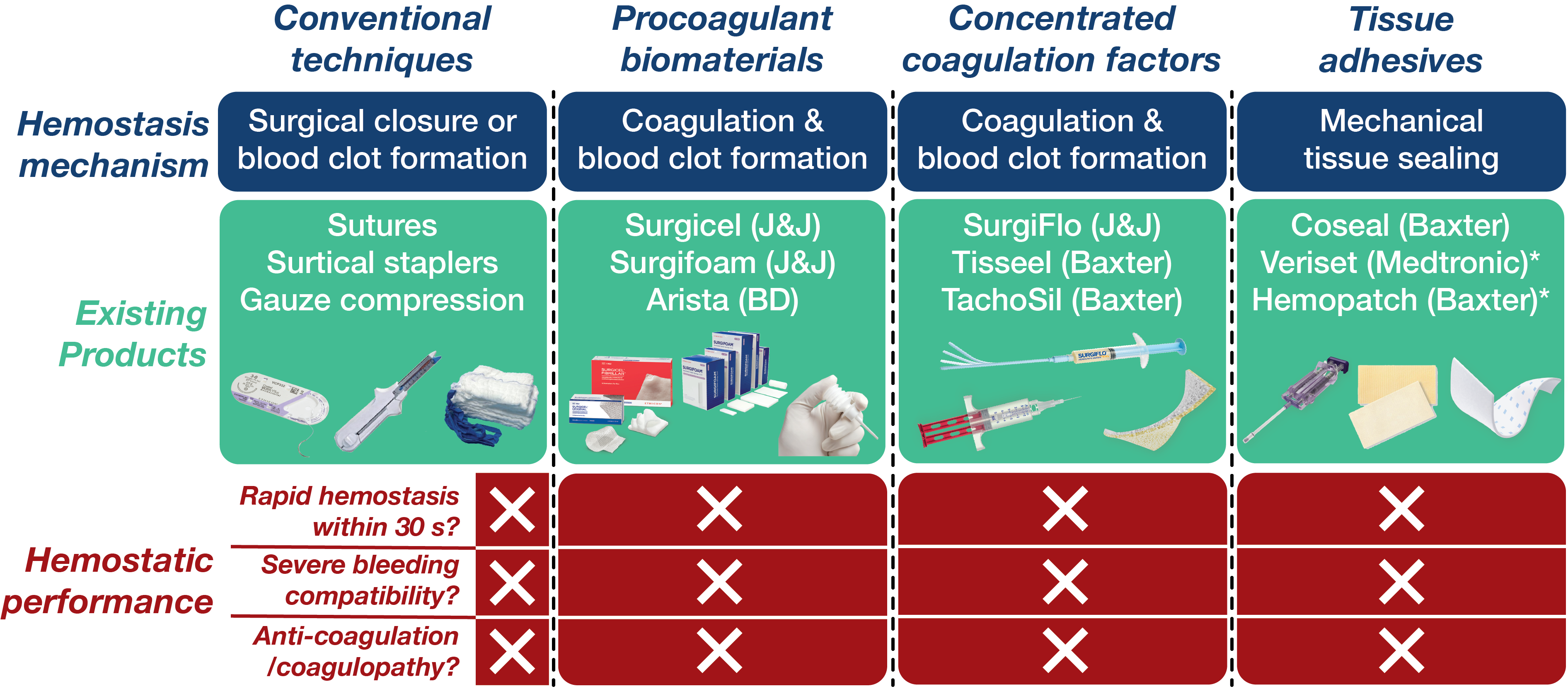
Existing hemostatic agents mostly rely on the promotion of inherent hemostatic mechanism - formation of blood clots or coagulation - based on various procoagulant biomaterials (gelatin, collagen, oxidized cellulose) or concentrated coagulation factors (fibrin, thrombin). However, blood clotting is an inherently slow process whose speed can further be slowed down in patients with blood thinners (heparin, wafarin) or coagulation disorders (coagulopathy). Furthermore, blood clots are mechanically weak and often easy to be broken down and washed away in severely bleeding wounds. Hence, there should be a new approach not relying on blood coagulation to address the limitations of existing hemostatic agents.
Nature-Inspired Solution
Our team has worked to develop better bioadhesives to address the limitations of existing tissue adhesives and sealants (for example, Dry double-sided tape for rapid adhesion on wet tissues and devices, Nature, 2019). But, hemostasis was a daunting problem to solve for us due to much more challenging characteristics than just sealing wet tissues and organs. Blood is not just fluid but contains a large number of blood cells and plasma proteins (actually blood's water contents are only around 55%), all of which serve as contaminants against the successful adhesion of tissue sealants. After months of searching for possible solutions, the silver lining came from an unexpected source. During the 2019 Gordon Research Conference on Science of Adhesion, I encountered interesting research on how a small marine crustacean - barnacles - can form robust adhesion on not only wet but also contaminated surfaces (Fears et al., Advanced Science, 2018).
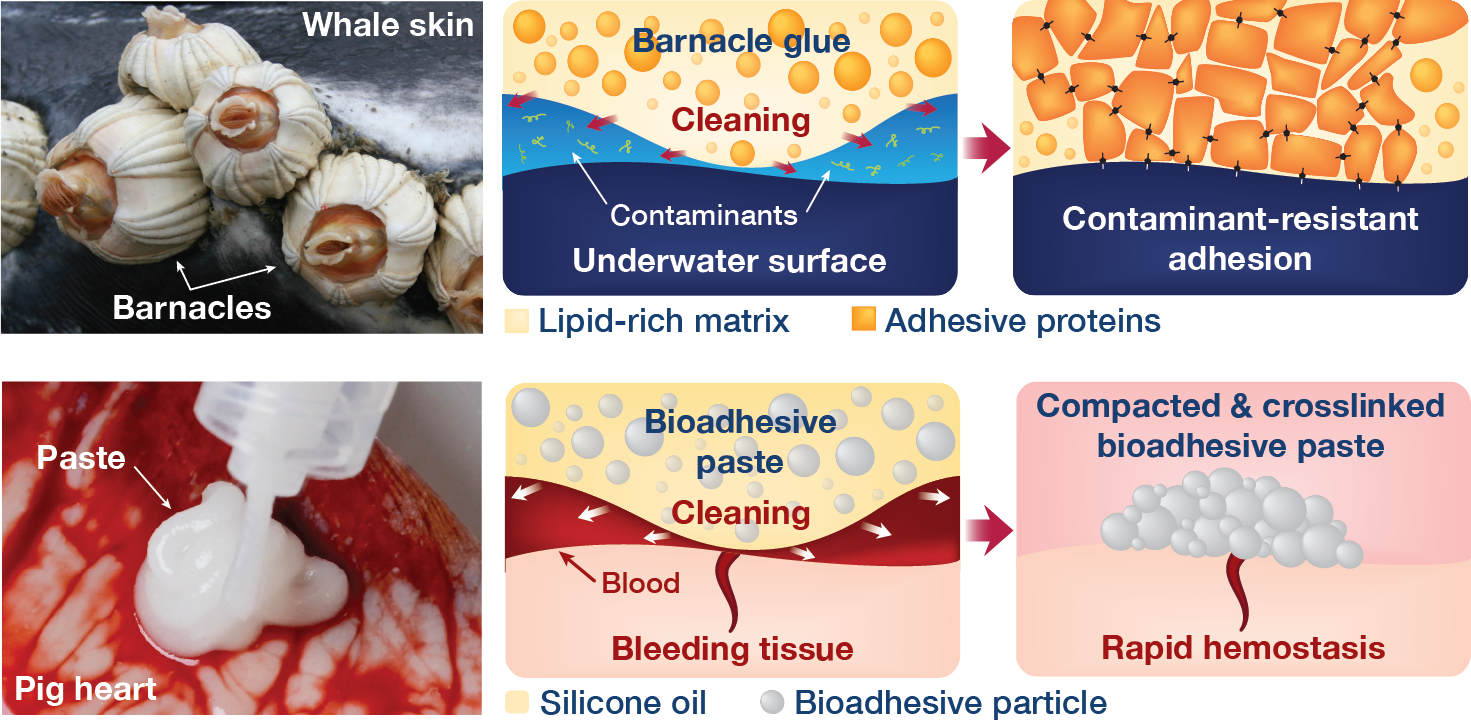
Mechanisms of barnacle glues and the barnacle-glue-inspired paste
Briefly, the barnacle's glue contains not only adhesive proteins but also lipid (or oil) rich components that can clean contaminants from the target surface to aid the adhesion formation. At first, it felt like just another interesting natural strategy in adhesion. But, after a bit of careful thought, the Eureka moment came - it was exactly the mechanism I have searched for to solve the challenges of hemostatic tissue sealing! After the excitement, I quickly took note in my phone with the link to the paper with a tag of "repel-crosslinking mechanism for hemostatic sealing", a name similar to the dry-crosslinking mechanism that I gave for the dry double-sided tape.
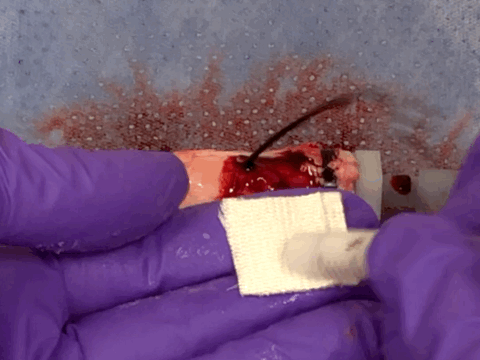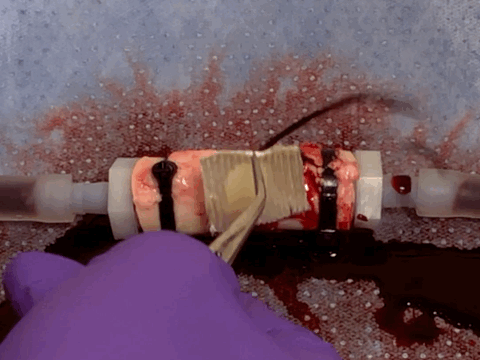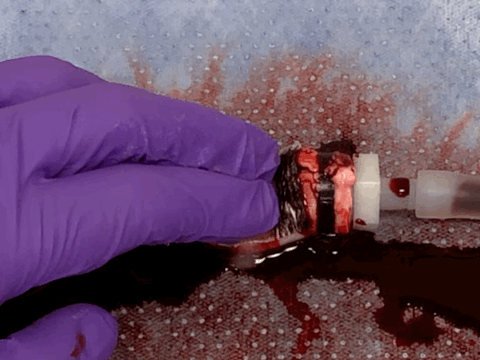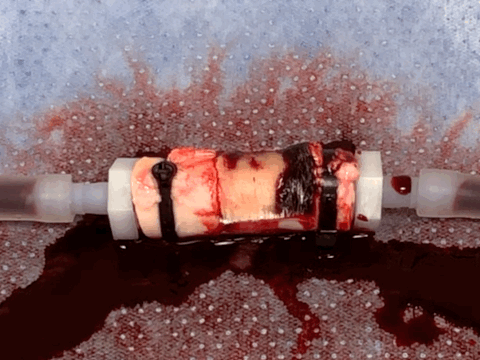
Rapid and coagulation-independent hemostasis by the paste
Returning from the conference to the lab, we worked hard to convert the barnacle-glue-inspired idea into a research project. Surprisingly, the process was pretty fast taking advantage of our accumulated expertise on bioadhesives as well as close collaboration with clinical experts at Mayo Clinic. Long story short, we developed a barnacle-glue-inspired paste consisting of dry bioadhesive particles suspended in medical-grade silicone oil, a carefully chosen selection of materials to mimic functions of barnacle-glue-components for contamination-resistant wet adhesion while minimizing risks in clinical translations from too new (or without a history of uses in medical devices/drugs) materials. As I test the barnacle-glue-inspired paste in various ex vivo and small animal in vivo hemostasis models, it quickly became my favorite invention - it worked amazingly well. In an ex vivo porcine aorta model with severe and fully anti-coagulated bleeding, I tried several best-performing (and expensive) commercially available hemostatic agents only to find that they do not work at all. However, the paste just sealed the bleeding aorta within 10 s in a single application, giving me a happy surprise (and our clinical collaborators loved the experimental video I took - we just all fell into love with the paste).
Toward Clinical Translation
Encouraged by ex vivo and small animal in vivo results, our collaborators at Mayo Clinic wanted to push further toward clinical translation by testing the paste on pre-clinical pig models. We chose a liver as a model organ which is one of the standard models for hemostasis. We further introduced heparin to the pigs to investigate the hemostatic perfomance for challenging fully anti-coagulated bleedings. After months of nervous preparation, our pre-clinical pig study was a great success. Anti-coagulated bleeding from a sizable liver defect could be sealed by a single application of the paste within 15 s whereas the market-leading fibrin patch (TachoSil®) could not achieve hemostasis of the same defect; all pigs survived with the favorable histological outcomes a month after hemostasis; and most impressively, our clinical collaborators at Mayo Clinic could do all these even though it was the first time they saw and used the paste!
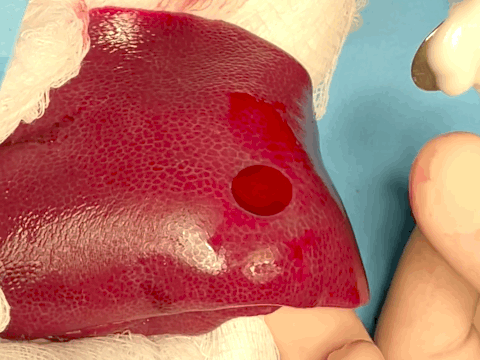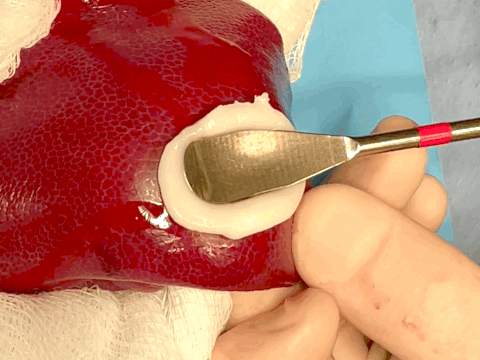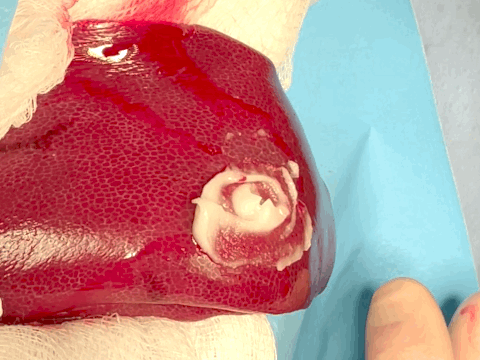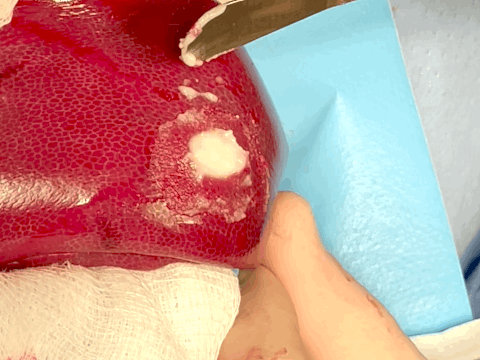
Hemostatic sealing of heparinized porcine liver bleeding within 15 s
Our team is now pursuing the next steps of clinical translation and commercialization including more clinically relevant large animal pre-clinical studies, technology transfer outside of the laboratory, and regulatory processes toward the FDA approval. We hope that we can see this exciting technology be used off the shelf to help patients in one day.
For more details, check out our paper “Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue” on Nature Biomedical Engineering.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in