Behind the Paper "On-surface synthesis of ballbot-type N-heterocyclic carbene polymers"
Published in Physics
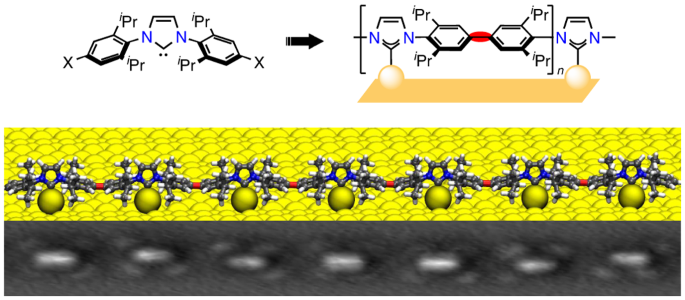
For the excellent international collaboration between National Center for Nanoscience and Technology, Institute of Physics Chinese Academy of Sciences, and University of Muenster, we go beyond monomeric NHCs and report on the synthesis of ballbot-type NHC polymers on metal surfaces. The novel physicochemical properties have been comprehensively studied via low temperature scanning tunneling microscopy, non-contact atomic force microscopy, X-ray photoelectron spectroscopy, first-principles calculations, and reactive force field simulations, explaining the high surface mobility of the incommensurate NHC polymers.
N-Heterocyclic carbenes (NHCs) have fostered considerable interest as attractive surface modifiers and anchors due to their strong σ-donating ability and the high tunability of the side-groups and surface mobility. Constructing the NHC based supramolecular structures, ranging from self-assembly to covalently connected architectures with ballbot configuration, on metal surfaces is highly desirable for catalysis and materials science but indeed challenging as it is driven by complex intermolecular interactions.
In an early work1, we rationally designed and experimentally realized unidirectional surface-anchored NHC rotor via utilizing the structural tunability of NHCs. This interesting work represents first real-space observation of a chiral-NHC-based rotor and open up new possibilities for construction of functionalized NHC systems with high catalytic applicability and superior stability. Recently, for large scale self-assembled NHC2, 3, the reversibly switched from a close-packed trimer phase to a chainlike dimer phase was achieved via the ring-flip of the cyclohexyl wingtip. Such controllable ratio of surface to volume in constructing patterned carbene structures is great importance for potential applications in catalysis.
After acquiring full knowledge towards design, synthesis, and experimental operation of organic molecule, we next set targets for realizing covalently linked polymeric NHC species to overcome the weak intermolecular coupling in self-assembled structures. Initially, the deposition of NHC polymer synthesized by a simple liquid-phase process is ruled out due to the carbonization of the polymer under high deposition temperature. We then chose the on-surface covalent reaction to get the clean and well-controllable NHC based polymers. Even though the Ullmann-type on-surface reaction has been extensively studied to gain fundamental understanding of covalently bound molecular polymers and to achieve various polymer-based technology applications, we stress, however, that transferring this technique to NHCs was anything but straightforward, largely due to the steric resistance effect in the ballbot configuration. Actually, we have tried several on-surface reactions to realize NHC polymerization. Only the Ullmann reaction shows high quality and controllable alignment in the ballbot-type NHC polymers. For example, NHC polymerization cannot be achieved by the coupling reaction of terminal alkynes, i.e. Glaser coupling reaction.
Beyond the synthesis aspect and controllable alignment of the ballbot-type polymers, the NHC polymers have also been characterized in unprecedented detail, e.g. interfacial charge transfer, quantitative measurement of lateral force. From a fundamental point of view, the direct high-resolution image of NHC polymers should offer a new avenue to fabricate and to even control NHC 2D networks at the nanoscale. From a technology viewpoint, the materials system represents an emerging class of nanoscale carbene structures with precisely tailored alignment. We thus believe that this study will greatly enhance the fabrication of carbene-based supramolecular structures and catalytic surfaces.
The full paper can be found here.
1 Ren, J. et al. A Unidirectional Surface-Anchored N-Heterocyclic Carbene Rotor. Nano Letters 20, 5922-5928 (2020).
2 Bakker, A. et al. An Electron-Rich Cyclic (Alkyl)(Amino)Carbene on Au(111), Ag(111), and Cu(111) Surfaces. Angew. Chem., Int. Ed. 59, 13643-13646 (2020).
3 Ren, J. et al. Reversible Self-Assembly of an N-Heterocyclic Carbene on Metal Surfaces. Angew. Chem., Int. Ed. 61, e202115104 (2022).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in