Bifunctional and Recyclable Polyesters by Chemoselective Ring-Opening Polymerization of a δ-Lactone Derived from CO2 and Butadiene
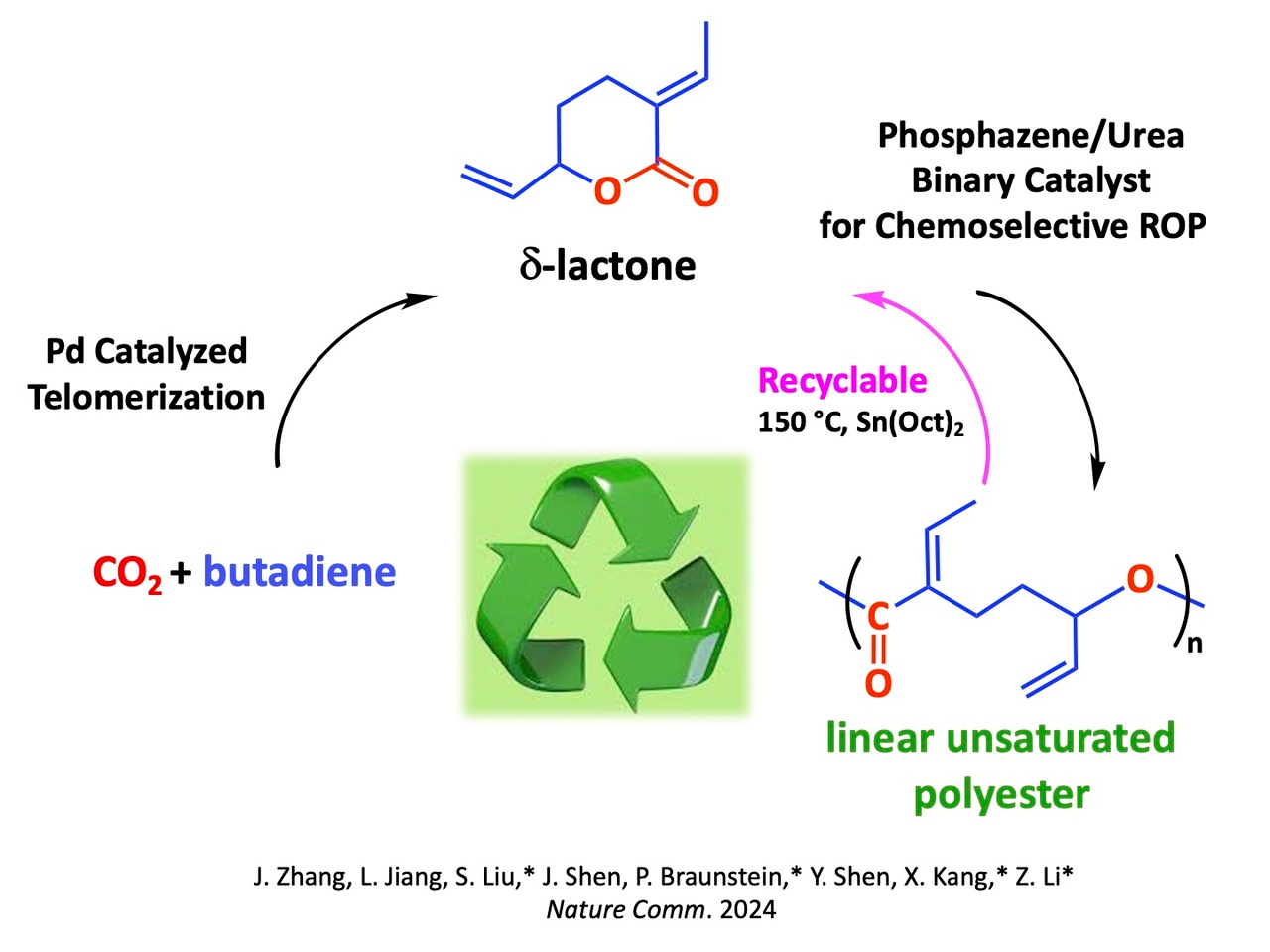
The direct utilization of carbon dioxide (CO2) to produce valuable chemicals represents a most relevant and timely objective and chemists from Qingdao University of Science and Technology, Qingdao (China), Dalian Medical University, Dalian (China) and the University of Strasbourg (France) have been able to produce bifunctional and recyclable polyesters by chemoselective ring-opening polymerization of the δ-lactone 3-ethylidene-6-vinyltetrahydro-2H-pyran-2-one (EVP), obtained by telomerization of butadiene and CO2. Although the selective ring-opening polymerization of EVP appears most attractive given the degradability and possible functionalization of the resulting aliphatic polyesters, all previous attempts have failed, owing to the stability of a six-membered disubstituted δ-lactone with low ring strain. Moreover, the conjugated double bonds could undergo Michael-addition reactions that would compete against or suppress ROP.
This δ-lactone has now been ring-opened using a phosphazene/urea binary catalyst, to afford exclusively a linear unsaturated polyester with a molar mass (Mn) up to 16.1 kg·mol-1 and a narrow distribution (Ð < 1.6).
The resulting unsaturated polyesters have a high CO2 content (29 wt%) and feature two different C=C double bonds per repeating unit, which can be sequentially and selectively post-functionalized to synthesize bifunctional polyesters. Thus, benzyl mercaptan was used as a representative thiol and reacted with poly(EVP)ROP in DMF at 20 °C in the presence of DBU as the catalyst. The selective functionalization of the internal alkene was confirmed by NMR spectroscopy and GPC of the resulting polyester. Next, the terminal alkene was reacted with isobutyl mercaptan via photoinduced thiol-ene click reaction in the presence of 2,2-dimethoxy-2-phenylacetophenone to install the second functional group and yield a bifunctional polyester. This stepwise strategy allows the preparation of a wide range of bifunctional polymeric structures, which are not accessible by ROP of the hydrogenated derivatives of EVP.
Finally, thermolysis of poly(EVP)ROP obtained by chemoselective ring-opening polymerization of the δ-lactone EVP can be fully recycled back to the pristine monomer, thus establishing a monomer-polymer-monomer closed-loop life cycle. In these polyesters, the CO2 content reaches 33 mol% (29 wt%). The reasons for this remarkable chemoselectivity were investigated by DFT calculations.
The article can be downloaded in free Open Access from : https://rdcu.be/dWmYg
Follow the Topic
Ask the Editor – Polymers
Got a question for the editor about Functional polymers? Ask it here!
Continue reading announcement



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in