
The coordination chemistry of beryllium is undergoing a renaissance. Despite its lightness, the chemistry of the Periodic Table’s fourth element is extremely poorly understood. This is, principally, because the high toxicity of the element and its compounds puts many researchers off. Nonetheless, there is growing acknowledgment that, with ample care, it is possible to work safely with beryllium. In summer of 2022, Prof. Simon Aldridge and I initiated a programme of research into the chemical properties of this unusual metal. We were motivated by a desire to fill in some of the gaps of our knowledge of the period 2 elements; beryllium sits between lithium and boron in the Periodic Table, bridging the metals and non-metals. We therefore hoped to be able to shed new light on the trends in bonding and reactivity across this series of light elements.
Our initial foray into beryllium chemistry was a collaboration with Dr Magnus Buchner at Philipps-Universität Marburg. For this work, we prepared a compound with an unsupported Be–Al bond via reaction of beryllocene (BeCp2) with a potassium aluminyl reagent, thereby yielding a complex with an [X2Al–BeCp] moiety. Considering the similar Pauling electronegativity of aluminium (1.61) and beryllium (1.57), this reaction could be thought of as a one electron oxidation of aluminium(I) and one electron reduction of beryllium(II). Thus, we anticipated that this hetero-metallic bonding linkage might electronically resemble the long-sought homo-metallic Be–Be bond. And indeed, an analysis of the electronic structure of this compound brought to mind diberyllocene (CpBeBeCp). Over the prior three decades, various researchers had come to the same conclusion: that diberyllocene should be stable. However, the first attempts to prepare this complex in a laboratory date back to the 1970s. Given our success preparing “half” of diberyllocene (i.e., the “neutral” [BeCp] fragment), we saw the full dimetallocene as our next synthetic target.
Previous attempts to prepare complexes with Be–Be bonds had been unsuccessful. We hypothesised that there were three principal reasons for this: 1) beryllium is a tiny metal – ligands need the right steric profile to kinetically stabilise of the Be–Be linkage, whilst not being so big that they prevent this bond from forming in the first place. Given previous theoretical studies, we believed that the unsubstituted cyclopentadienyl ligand would afford the Be–Be bond sufficient kinetic stability; 2) beryllium–halide interactions are very strong, due to the powerful Lewis acidity of Be2+. Hence, we anticipated that reduction of alternative beryllium(II) starting materials (i.e., organo-beryllium species) might be more straightforward; 3) we also reasoned that widely-employed alkali metal reductants might be too powerful, turning beryllium(II) straight into beryllium metal. The question of the correct reductant, however, remained unanswered…
Here, we turned our attention to beryllium’s heavier homologue, magnesium. The seminal report of magnesium(I) dimers in 2007 initiated the flourishing field of low-oxidation state alkaline earth metal chemistry. It had been calculated that Be–Be bonds would be significantly stronger (by around 50%) than Mg–Mg bonds. Therefore, we hypothesised that it might be possible to leverage this to reduce beryllocene, yielding diberyllocene. And this works. We assume the reaction initially forms a beryllium(0) complex with a Mg–Be bond, before this complex engages in comproportionation, reducing a second equivalent of beryllocene and forming diberyllocene, along with two equivalents of a magnesium(II)-cyclopentadienyl complex.
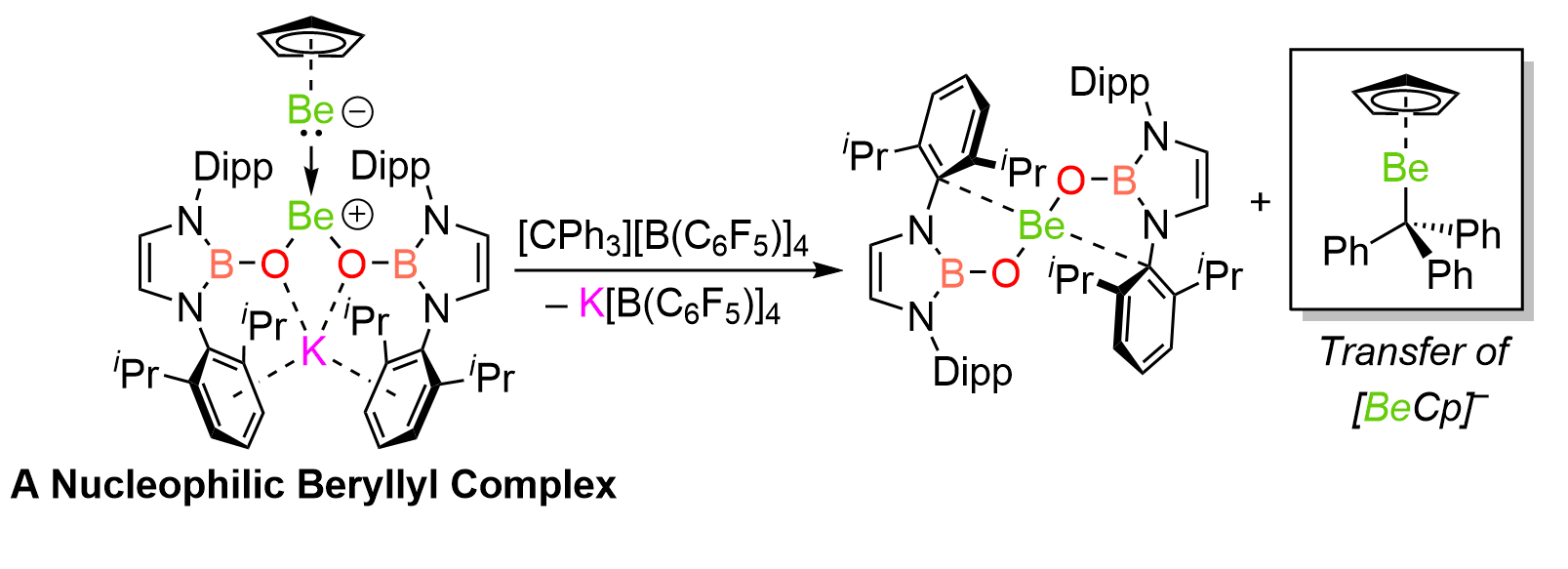
With diberyllocene in hand, we wanted to see if we could start to draw comparisons between the homo-elemental bonds of beryllium and boron - “neighbours” in the periodic table. The first results are outlined in this Nature Chemistry article. Here, we prepare some new complexes with Be–Be bonds and find that there are remarkable similarities between the bonding and reactivity of diborane(4) reagents and their beryllium analogues. It is possible to polarise the Be–Be bond, just like the B–B bond, by coordinating more anionic ligands to one Be centre than the other. This puts one in mind of sp2-sp3 diboranes, which act as sources of the boryl anion. And indeed, we find that one of our unsymmetrically-coordinated “diberyllanes”, prepared here, reacts as a source of the “beryllyl” anion, which formally features a beryllium(0) centre.
From here, we look forward to further probing the reactivity of the Be–Be bond, examining if this mimics, or diverges from, that of the B–B bond.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in