Born to be soft
Published in Materials

There is no doubt that the science of new porous materials called PCP (Porous Coordination Polymer) or MOF (Metal-Organic Framework) of infinite and periodic linkage among metal ions and organic molecules is flourishing wildly today. This is evident from the fact that the flagship conference in this field, MOF2024, held in Singapore this July, was a great success with more than 1,000 participants. We can say that what triggered the expansion of the current PCP/MOF field should be the discovery that they could reversibly adsorb a significant amount of gas to be applied as porous solids like zeolites and activated carbons. Our research group of Professor Susumu Kitagawa played this role with a report in 1997 on a neutral framework with a “tongue-and-groove” structure. Around the same time, Professor Omar Yaghi reported that another neutral porous crystal named MOF showed equivalent gas-adsorbing performance. Since then, a vast variety of PCP/MOFs have been synthesized worldwide. When one talks about PCP/MOFs, which has grown into a vast field of functional materials science, it might be allowed to say that our work in 1997 is one of the essential source streams. This “front” history is probably familiar to many relevant researchers.
For a long time, we had found something strange about the gas adsorption behavior of our first PCP. Looking at its crystal structure, the pores of the tongue-and-groove PCP were shaped like the edamame pods, and the constricted part was smaller than the size of the gas molecules to be adsorbed (Figure 1(c)). Also, at the time of the discovery of the PCP, it was commonly believed that the framework structure of porous materials should be “rigid,” not “flexible/soft” at all. As a result, the question of how gas molecules pass through the constrictions of the pores and enter the interior of PCPs to fill the entire pore space remained a mystery, and there was no means and experience to clarify this. Indeed, it was not until a bit later than the first report that we could recognize the mystery precisely! For, we could only capture the pore structure quite roughly since we did not have any software like Mercury, CrystalMaker, etc., to draw crystal structures based on the van der Waals atomic radii.
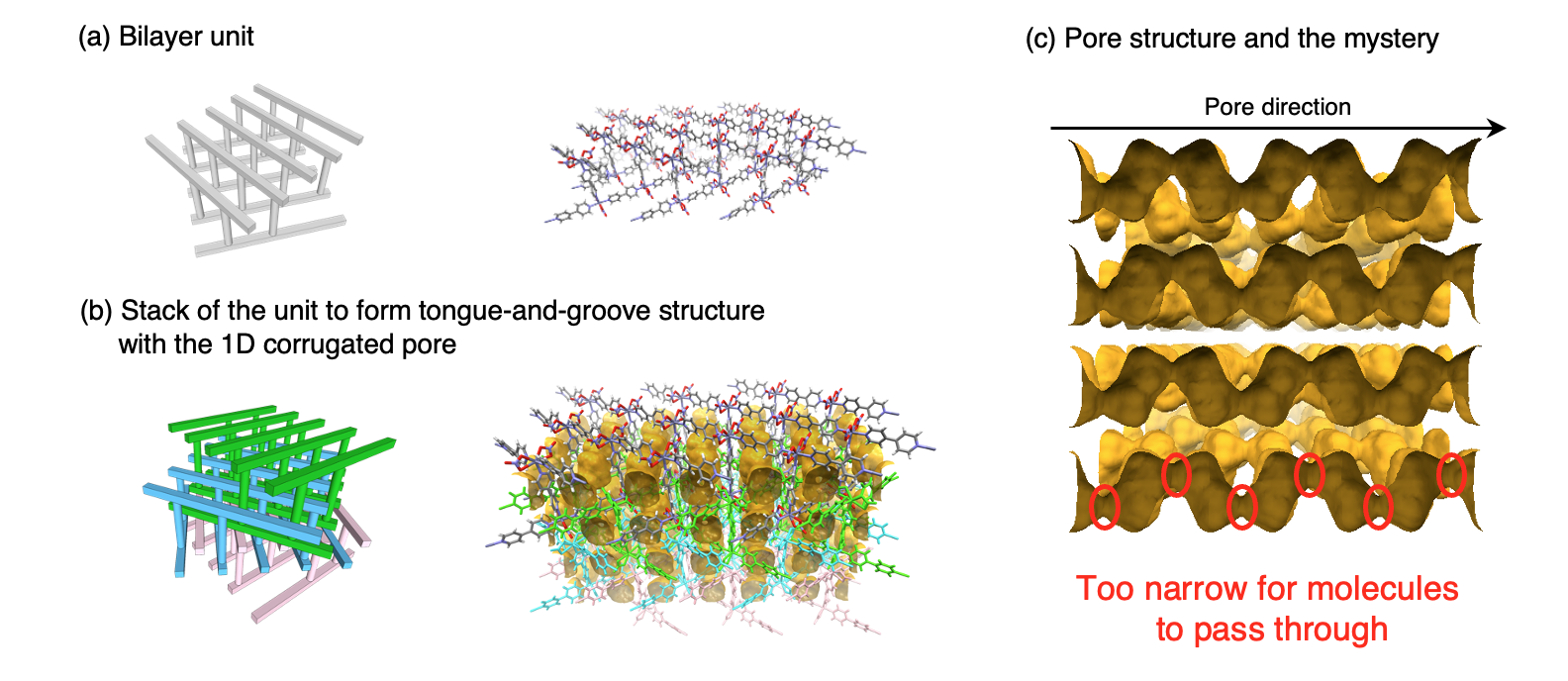
Figure 1. (a)The structure unit of our first PCP reported in 1997. (b)Between tongue-and-groove unit layers, the 1D corrugated pore (edamame-pod shape) inside is depicted on the yellow surface. And the long-time mystery of this PCP is solved in the article.
We once tried to solve this mystery 20 years ago when I (H.S.) was a graduate student of Prof. Kitagawa, co-corresponding author of this article. At that time around the early 2000s was a period that could be called the renaissance of PCP/MOF research, starting with “tailor-made framework construction with a modular strategy,” basic concepts that are still the most important today, such as “secondary building unit,” “permanent porosity,” and so on, were established one after another. Looking back on this period, Prof. Kitagawa says, “These were extremely exciting days as a researcher, making a new concept no one has ever come up with.” Among these, our group was beginning to develop the concepts of “in-situ analysis of gas adsorption states” and “flexible/soft porous frameworks.” At the time, to further verify and advance these concepts, we expected that the introduction of gas would push apart the interdigitating parts of the tongue-and-groove structure, and we tried to capture the global structural transformation using powder diffraction with synchrotron radiation X-ray, which we had started to use at the time. Unfortunately, the investigation had been suspended for several reasons without sufficient evidence being obtained, and I moved on to another research topic. Looking back, I think the sample handling was poor without considering its framework transformation in the presence of atmospheric moisture, which has been revealed in this work. Anyway, following a famous quote, it was “whereof we cannot speak, thereof we must be silent.”
After obtaining my degree, I spent more than ten years outside the PCP/MOF field, but by a twist of fate, I had the opportunity to work with Professor Kitagawa again in 2022. I was surprised to see how much the laboratory had become more extensive and sophisticated while I was away. There is enough gas adsorption apparatus, so there is no longer a need to wait several months to obtain a single isotherm! What is more, in-situ single crystal X-ray diffraction under a specific gas adsorption state is at command in the laboratory, developed and managed by my current colleague, Dr. Ken-ichi Otake, also a co-author of the article. Meanwhile, diffusion by local dynamic motion (DLDM) also matured around 2020, and knowledge has been accumulated to understand the phenomenon of molecules passing through narrow pore spaces in various situations. Everything I had needed 20 years ago was in the right place for us. Then, in the early summer of 2023, by chance, we received an invitation to a themed collection in Communications Materials. The collection title was “Reproducibility in well-defined porous frameworks,” which we thought was a perfect opportunity to present our solution to the mystery. The time was ripe for us to take revenge, and we began to work on the content of this paper in earnest because this was a problem that absolutely must be solved, as it concerns our research identity.
Although I was a little confused about how to reproduce the synthesis of the PCP, which I had last done a long time ago, it didn't take long to get my intuition back, and the data collection was generally smooth. Without any difficulty, we could obtain the gas adsorption isotherms over the entire range of relative pressures at the boiling point of the adsorbed molecules, which was not possible in 1997. Our tongue-and-groove PCP adsorbs a substantial amount of gases well and consequently has permanent porosity. Reproducibility check done with no problem. Prof. Kitagawa immediately shared these preliminary data with his research communities. Although the colleagues might wonder why he talked about the oldest PCP, the feedback was positive, with not a small surprise. However, we were beginning to notice another strange thing at that point. CO2 adsorption isotherm at 195 K showed a two-step profile with abrupt uptake at a certain pressure, indicating that this PCP could be a “soft PCP,” as we had once expected.
Therefore, we considered obtaining the crystal structure of each stage of CO2 adsorption in this PCP by entirely using the “in-situ measurement” technology of single crystal X-ray diffraction. First, the structure of the PCP crystal heated in a vacuum before the adsorption molecules were introduced showed no solvent molecules in the pores. This clearly indicates that the solvent molecules passed through the pores, which are narrower than the molecules themselves, without destroying the framework structure and were released outside due to heating and vacuuming. Next, CO2 was introduced under conditions before adsorption reached saturation. At that time, the structure of the PCP framework and pores was almost the same as the empty state before adsorption, but it became clear that CO2molecules were filling the cavity of the “bean,” which was initially filled with solvent. It was confirmed that the mystery phenomenon was occurring, as CO2 molecules were passing through the narrower part of the crystal and entering the pores. However, these observations do not explain how this penetration was made possible.

When diffraction measurements and structural analysis were carried out under conditions where more CO2 was adsorbed onto the PCP crystals, a crystal structure was obtained that was slightly different from the previous one. The extra CO2 molecules that had entered the pores had caused the part corresponding to the narrow part of the pores to deform (the torsion angle of the 4,4'-bipyridine had changed), and the pores had widened to the extent that CO2 could pass through them. This corresponded beautifully with the behavior (abrupt uptake and its amount) of the stepwise adsorption isotherm. In this local region, when adsorption has not progressed sufficiently, there is freedom of movement, and it can be thought of as being like a “half-open door,” with gas molecules entering the crystal by pushing it open each time. We have named this mechanism, in which the door is pushed open each time, “squeeze adsorption” based on the local motion flexibility. In addition, since the deformation of this local structure extends to the entire crystal lattice in the saturated adsorption state, we have also found that the second step in the CO2 adsorption isotherm is typical “soft PCP” behavior involving a structural phase transition. Our PCP was proved to be indeed soft, both locally and globally, although the origin of softness was different from what we had initially expected.
Through these, we have solved the long-time mystery of PCPs that exhibit gas adsorption. At the same time, we discovered that the “first gas-adsorbing PCP” was also the “first soft PCP.” This discovery is a historical twist that suggests that we had soft PCPs in our hands even before they were predicted in 1998 by Professor Kitagawa. It is a significant result that could redefine the origins of PCP research. PCPs have always been soft since before their first appearance in this world. Days after this paper was published, I asked Prof. Kitagawa, “Did you have the concept of soft porous crystal in mind back in 1997?” He answered, “I had its image dimly in my mind. I thought that this phenomenon must exist, but it was not clear which organic molecules and metal ions would produce a framework that showed flexibility. But I did not expect this PCP to be the one”. We were digging near a gold mine without realizing it. A good lesson from this project is that “In the field of observation, chance favors the prepared mind.” ― Louis Pasteur. And we cannot even prepare for what we have not imagined at all. Then we need imagination for what has not appeared yet to be a real pioneer in a new essential field in science, i.e., we should always be “flexible/soft” for everything, no matter whether it substantially exists or not at that time. Yes, like these PCPs.
(Cover art: ©Mindy Takamiya/iCeMS, Kyoto University)
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Multifunctional hydrogels
Publishing Model: Open Access
Deadline: Feb 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in