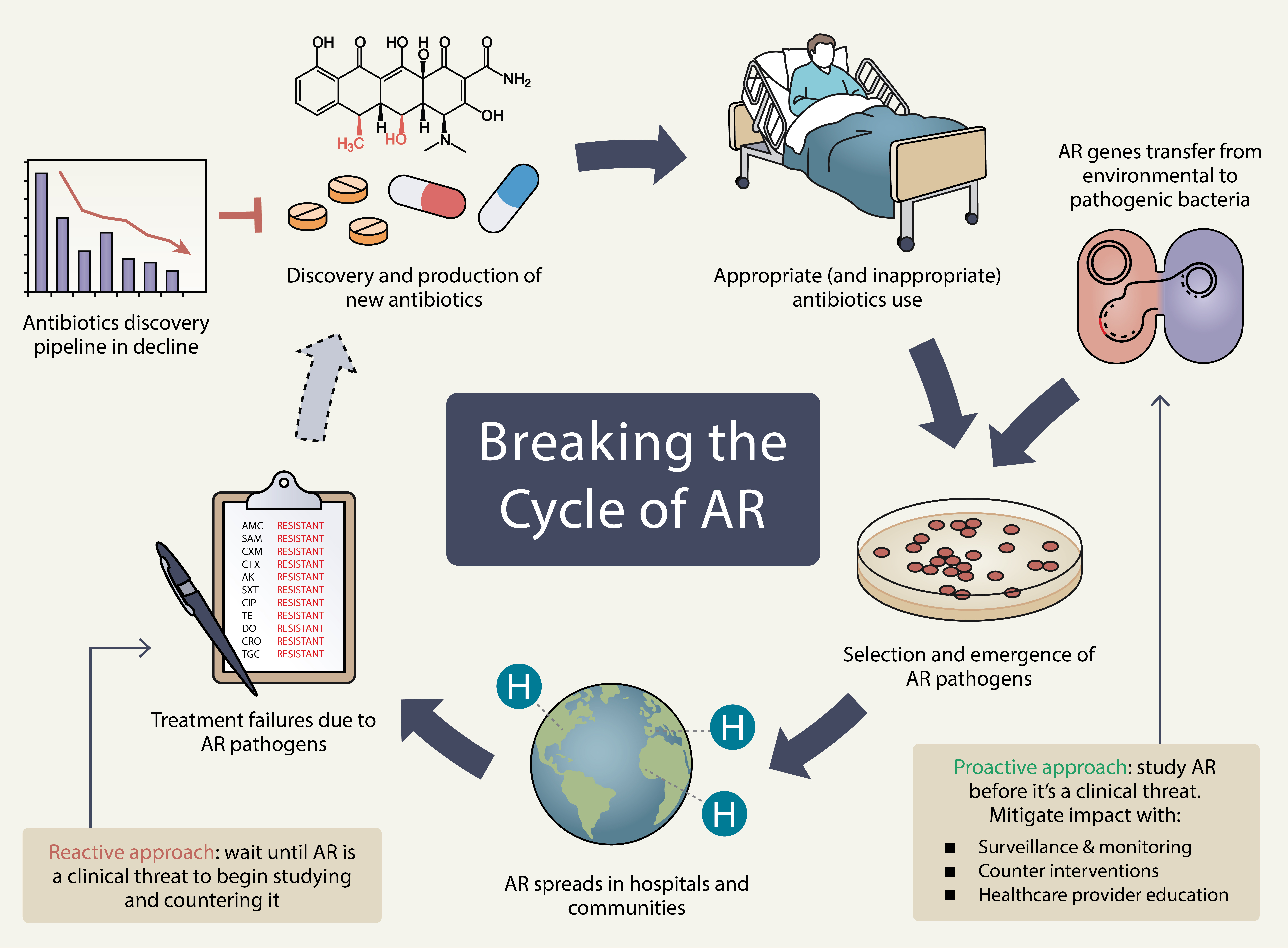
Breaking the Cycle of Antibiotic Resistance
Published in Microbiology

Modern medicine is built upon a foundation of antibiotics. Since the production of penicillin in 1942, antibiotics have helped reduced deaths from infectious diseases by 70%, and once unthinkable treatments, including joint replacements and chemotherapies, have become routine. However, the effectiveness of antibiotics has been steadily and severely undermined by antibiotic resistance (AR). The deployment of each new antibiotic has been quickly followed by the emergence of pathogens resistant to their effects. The predictability of AR is such that we could amend the famous Benjamin Franklin quote to, “nothing is certain except death and taxes and antibiotic resistance.” In past decades, we could count on the discovery of new antibiotics to replace those AR rendered useless—restarting the cycle of AR. But with fewer new antibiotics being discovered each year we can no longer afford this kind of reactive approach. Instead, protecting our therapeutic arsenal requires we be proactive: studying new AR mechanisms before they become a clinical threat.
Microbiology has myopically studied AR mechanisms that had already emerged in pathogens. But we now know that, in addition to the gradual processes of mutation and natural selection, pathogens can rapidly become resistant by acquiring ready-made genes from non-pathogenic bacteria via horizontal gene transfer. Recent advancements in DNA sequencing have made it possible to discover AR in environmental and human gut microbiomes. Our group has used these technologies to proactively study an emerging family of antibiotic-inactivating enzymes: the tetracycline destructases (DES).
First discovered in 1948, tetracycline antibiotics are widely used in the clinic and agriculture. To resist these drugs, bacteria employ dozens of efflux pumps (EFF), ribosomal protection proteins (RPP), and DESs. EFFs have caused resistant infections since 1953, and RPPs since 1980, but the DESs have historically been absent from pathogens. That is no longer the case: In 2013, DESs were first discovered in hospital isolates and quickly spread to four of the six ESKAPE pathogens—the leading causes of hospital-acquired infections. In the blink of an eye, DESs went from uncommon oddities to emerging clinical threats. Today, there are more than 30 characterized enzymes from two clades (DES1 and DES2).
For over 10 years our interdisciplinary team worked to understand, predict, and mitigate DES resistance. We have discovered new DESs from soil and human gut microbiomes, elucidated their mechanism of tetracycline inactivation, and developed small-molecule inhibitors against them. My specific contributions include: discovering new genes and identifying residues critical for function (1), validating anhydrotetracycline analogs that block DES activity (2-4), and developing a new genomics technology to reconstruct the history of the tetracycline resistome (5).
The growing number of DES protein sequences permitted a deep investigation into DES sequence determinants of function. Since residues critical for function tend to be conserved across protein families, we focused on the 31 amino acid positions 100% conserved across all DESs. Using profile hidden Markov models, which generate a position-specific scoring system based on sequence conservation, we searched >300 million sequences for cryptic DESs. Screening 50 high-scoring hits by recombinant expression in Escherichia coli, revealed 14 as new DES enzymes. Next, we evaluated how these 31 residues might contribute to AR activity. Mutations at 5/31 positions completely abolished resistance to all tetracyclines, proving they’re essential to activity. Mutations at the other positions had variable impact on cell and in vitro activity, structure, and stability. This study expanded the diversity of DES sequences and provided valuable insights into the role of important residues (1).
However, as we rushed to learn more about how DESs worked, a question kept coming to mind: Why were DESs only just now emerging, >60 years after the discovery of EFFs? A clue came while researching the history of tetracycline drug development. Shortly after the deployment of generation 1 tetracyclines (1948-1953) EFFs were discovered in 1953. After gen 2 tetracyclines were discovered (1961-1967) came RPPs in 1980. Lastly, with gen 3 tetracyclines (1993-present) we have the emergence of DES1s in 2013. This suggested that each generation preferentially selected for a specific resistance mechanism, leading to their expansion in bacterial populations and eventually emergence in human pathogens.
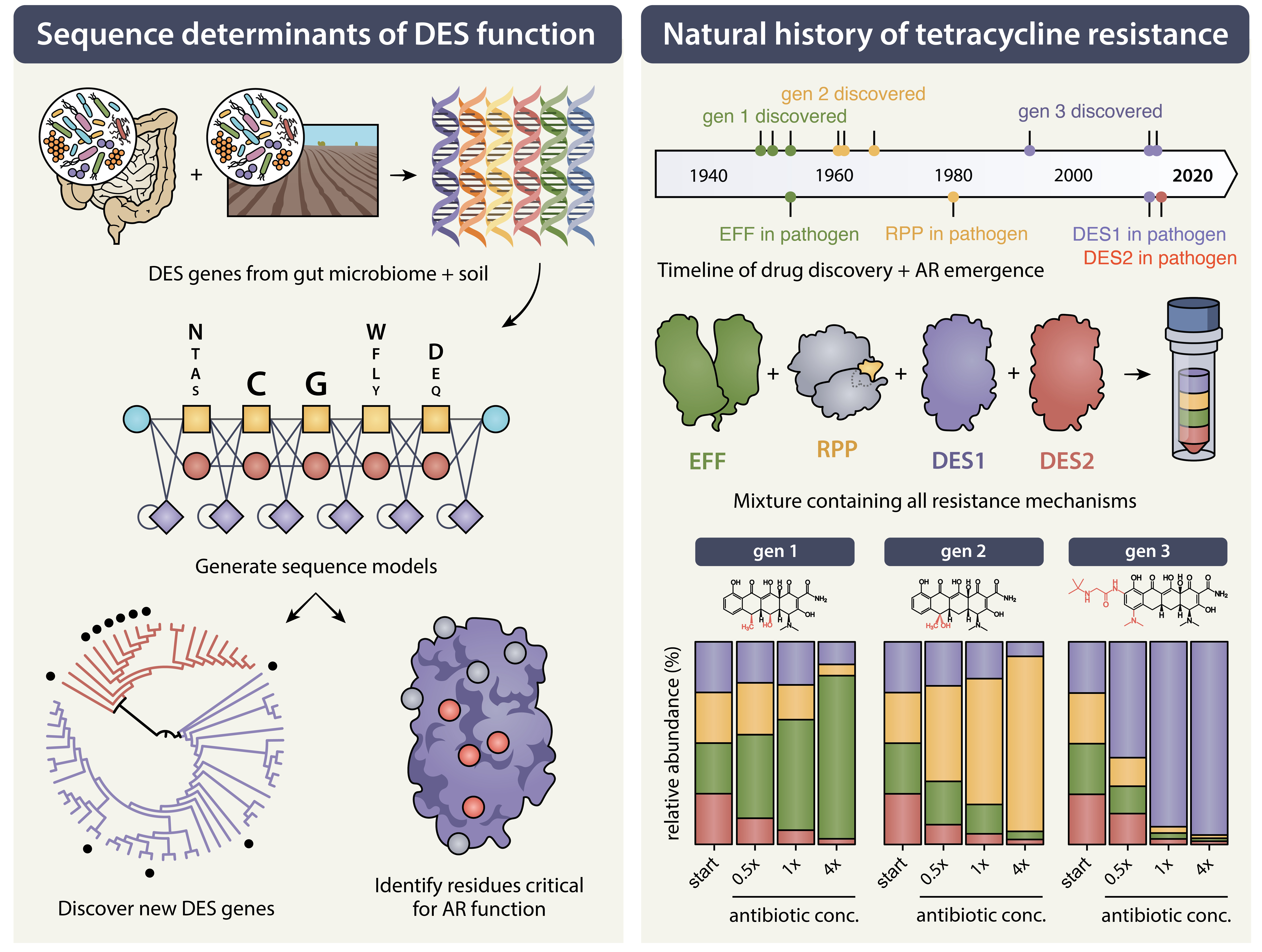
We aimed to test this directly. In effect, to reconstruct the natural history of tetracycline resistance in a test tube. We constructed 24 strains of E. coli, identical except they encoded a different tetracycline resistance gene (6 EFFs, 6 RPPs, 6 DES1s, and 6 DES2s). Pooling these at equal relative abundances, we created a synthetic bacterial population. To quantify the relative abundance of these near-identical strains in mixed cultures before and after growth in antibiotics we developed CompAReSeq, a sequencing method which counts strain-specific DNA barcodes. First, we grew this mixture in a gen 1 tetracycline. After 20 hours of growth the EFF strains had expanded from 25% to 80% of the population. When that same mix was instead grown with gen 2 tetracyclines, RPPs expanded to 90%. Lastly, with a gen 3 tetracycline, DES1s expanded to 95%. This dynamic was confirmed to be primarily driven by mechanism and drug generation, not by individual strains’ minimum inhibitory concentrations or growth rate defects. This conclusively showed that each generation selects for specific AR mechanisms, and that this generation-mechanism pairing matches the history of tetracycline resistance in pathogens (5).
These studies have uncovered vulnerabilities in DES biology that can be exploited by non-antibiotic interventions. By studying DESs before they’re a clinical threat, we have finally broken the cycle of drug discovery and AR. In the two-millennia-old Canon of the Yellow Emperor, a court physician advised: “to administer medicines to diseases which have already developed . . . Is comparable to the behavior of those persons who begin to dig a well after they have become thirsty.” We will always be behind AR if we wait until it’s already in the clinic to develop strategies to mitigate its spread. This inevitability makes AR an existential threat but if we adopt a proactive approach, it may also be the key to finally getting ahead of AR.
References
- Blake KS, Kumar H, Loganathan A, Williford EE, Diorio-Toth L, Xue YP, et al. Sequence-structure-function characterization of the emerging tetracycline destructase family of antibiotic resistance enzymes. Commun Biol. 2024 Mar 16;7(1):336.
- Williford EE, Xue YP, Tang WK, Li R, Jones KV, Blake KS, et al. C10-Benzoate Esters of Anhydrotetracycline Inhibit Tetracycline Destructases and Recover Tetracycline Antibacterial Activity. ACS Infect Dis. 2025 Feb 6;acsinfecdis.4c00912.
- Williford EE, DeAngelo CM, Blake KS, Kumar H, Lam KK, Jones KV, et al. Structure-Based Design of Bisubstrate Tetracycline Destructase Inhibitors That Block Flavin Redox Cycling. J Med Chem. 2023 Mar 23;66(6):3917–33.
- Kumar H, Williford EE, Blake KS, Virgin-Downey B, Dantas G, Wencewicz TA, et al. Structure of anhydrotetracycline-bound Tet(X6) reveals the mechanism for inhibition of type 1 tetracycline destructases. Commun Biol. 2023 Apr 17;6(1):423.
- Blake KS, Xue YP, Gillespie VJ, Fishbein SRS, Tolia NH, Wencewicz TA, et al. The tetracycline resistome is shaped by selection for specific resistance mechanisms by each antibiotic generation. Nat Commun. 2025 Feb 7;16(1):1452.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in