Bridging the Gap: Enhancing Diversity in Clinical Trials to Advance Medicine
Published in Social Sciences, Biomedical Research, and Public Health
Achieving breakthrough discoveries to advance human health requires clinical trials that include communities who have historically been less engaged in the research and development of potentially life-saving medical interventions.
Change, however, is possible. A broad coalition has developed a practical approach to strengthening diversity in clinical trials, as detailed in our recent publication, Supporting diversity in clinical trials: the Equitable Breakthroughs in Medicine site maturity assessment model, published in Trials. This model represents a new way of addressing systemic inequities in clinical research.
A Growing Necessity
Historically, lack of diversity in clinical trials has been attributed to barriers participants may face like limited access to trials in their communities, the financial and time burden, hesitancy due to lack of information, disinformation or misinformation, or more often simply not being asked to participate.
Equitable Breakthroughs in Medicine Development (EQBMED), funded by a grant from PhRMA, seeks to ensure communities of color and rural residents have access to innovative clinical trials so that those who may wish to participate in trials have opportunities to do so.
The Current Landscape
Clinical trials should reflect the intended treatment population for a medicine, but that is not always the case. When the participant pool does not reflect the diversity of the population it can reinforce mistrust in clinical research.
Diverse clinical trials can also provide researchers more information about the patient experience with a potential new medicine, potentially highlighting different responses to the medicine, and helping to further inform the safety and effectiveness profile of that medicine for patients.
A Systematic Approach: The EQBMED Model
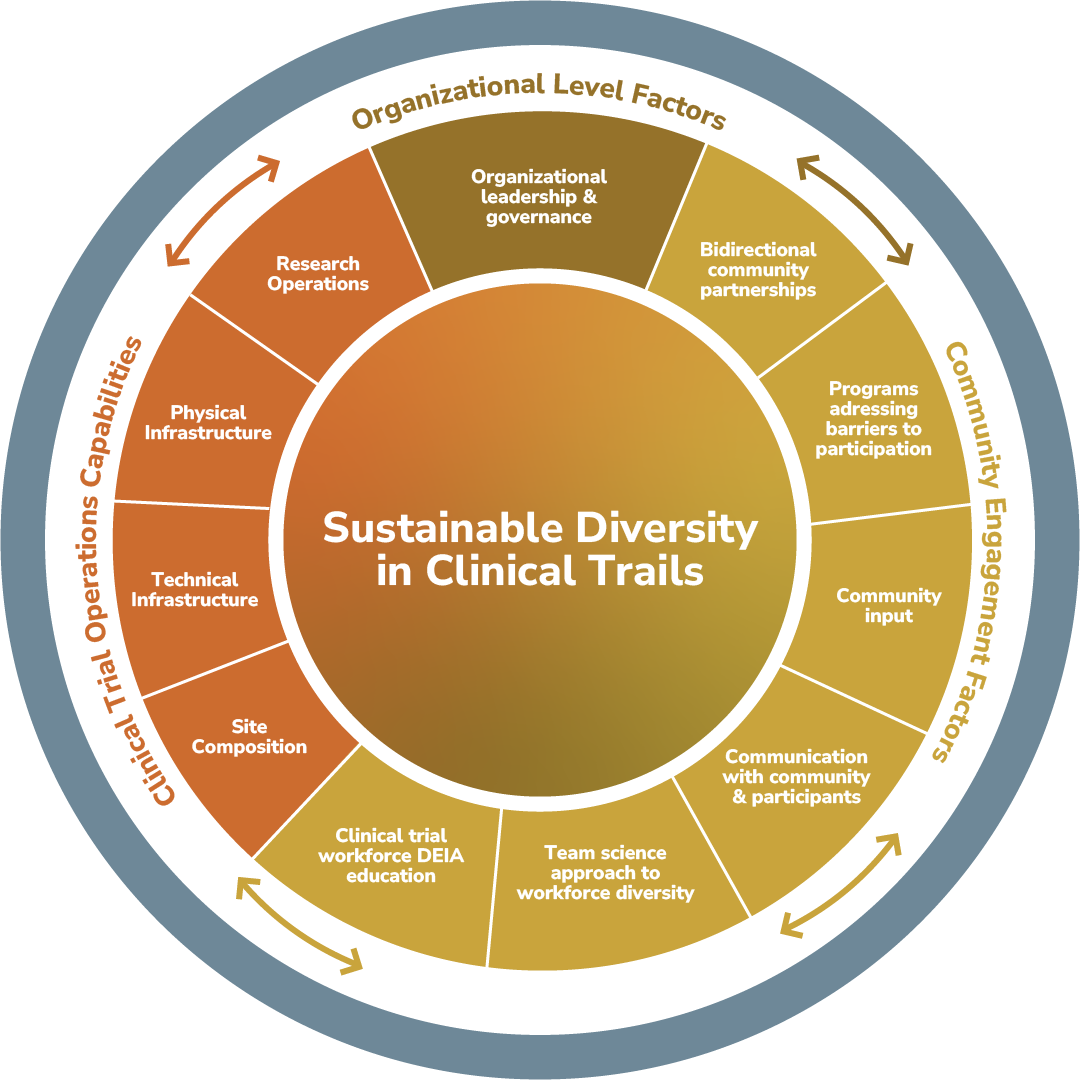
The EQBMED Site Maturity Assessment Model adopts a dual focus: enhancing clinical trial infrastructure and fostering meaningful community engagement. Developed over two years with input from diverse stakeholders, including pharmaceutical leaders, healthcare providers, and community advocates, the model integrates:
- Research Operations: This pillar evaluates organizational readiness, resource allocation, and procedural efficiency to support industry-sponsored trials (Fig. 1).
- Community Engagement: Recognizing the critical role of trust, this component emphasizes partnerships with local leaders and community-based outreach efforts (Fig. 1).
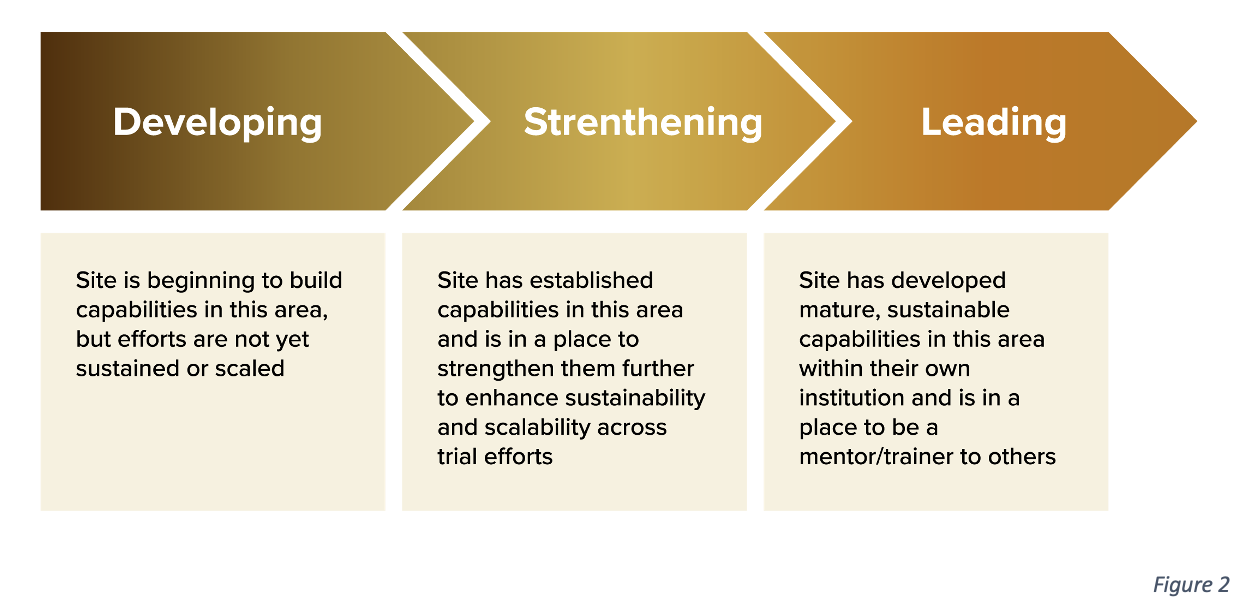
The model employs an iterative, site-driven methodology. It assesses a site’s current capabilities, identifies gaps, and prescribes actionable steps toward achieving “maturity”—defined as the ability to conduct inclusive, high-quality clinical trials (Fig. 2). Key innovations include:
- Incorporating social determinants of health into trial design.
- Training healthcare professionals to address participants’ cultural and logistical needs.
- Establishing feedback loops to adapt to evolving community expectations.
Central to the EQBMED Site Maturity Assessment Model is its emphasis on meaningful community engagement. Building trust is critical to increasing participation in clinical trials, particularly among communities that have faced historical mistreatment, such as during the U.S. Public Health Service Syphilis Study at Tuskegee. This legacy has understandably created a lasting mistrust of medical research among minority populations.
The EQBMED approach focuses on fostering open communication and building partnerships with community leaders and organizations, who have the collective knowledge, valued trust, and deep community ties to support the creation of an accessible landscape in medical research. By involving communities in the design and implementation of clinical trials, the model ensures that their voices are not just heard but are integral to the process.
As the EQBMED initiative articulates, community engagement is key to overcoming mistrust and ensuring that research benefits are shared equitably across all populations.
Lessons from Implementation
The effectiveness of the EQBMED Site Maturity Assessment Model has been demonstrated through its pilot implementation at various clinical trial sites. For example, one site introduced community health workers who understood the cultural nuances of the local population. These trusted intermediaries improved recruitment and retention rates by addressing community-specific concerns and fostering trust.
At another site, hosting informational sessions in community centers proved to be a successful strategy. These sessions provided clear, accessible information about the trials’ purpose, procedures, and potential benefits, demystifying the process and creating a platform for open dialogue.
These examples highlight the importance of addressing both structural barriers, such as infrastructure and staffing, and perceptual barriers, like mistrust and lack of awareness. By doing so, the EQBMED Site Maturity Assessment Model helps create an inclusive research environment where diversity is not an afterthought but a foundational element.
Charting a Path Forward
Achieving diversity in clinical trials is more than a logistical challenge; it is a moral imperative. As we stand on the brink of numerous medical breakthroughs, we must ensure that no demographic is left behind. The stakes are too high to ignore.
The EQBMED model offers a clear roadmap for building inclusive clinical trial practices. By committing to these practices and championing diversity at every level, we can ensure that the benefits of medical advancements are shared equitably, regardless of race, ethnicity, geography, or socioeconomic status.
The journey toward diverse clinical trials is complex, but it is one we must undertake. By fostering trust, enhancing community engagement, and implementing targeted strategies, we can bridge the gap and pave the way for true medical advancements that reflect and serve our diverse society. Enhancing diversity in clinical trials is not just about improving research outcomes; it is about upholding equity in medical science. Together, we can create a future where everyone’s health and well-being are equally valued and safeguarded.
Follow the Topic
-
Trials

This journal encompasses all aspects of the performance and findings of randomized controlled trials in health, including articles on general trial methodology and trial processes, study protocols and statistical analysis plans for randomized controlled trials, commentaries and traditional results papers.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in