Caffeine prevents alcohol-induced activation of the mesolimbic dopaminergic pathway
Published in Neuroscience, Protocols & Methods, and General & Internal Medicine

Caffeine and alcohol are the most consumed psychoactive substances worldwide (1, 2), profoundly impacting billions of lives. Recent findings from our group demonstrate that low doses (3mg/kg and 15 mg/kg) of caffeine significantly inhibit ethanol-induced conditioned place preference and alcohol-triggered extracellular signal-regulated kinase phosphorylation (pERK) in the Shell subregion of the Nucleus Accumbens (AcbSh) (3). Both behavioral and biochemical effects are closely regulated by the Mesolimbic Dopaminergic Pathway, a brain circuit mediating the reinforcing properties of alcohol and other addictive substances (4, 5). Building on this, we investigated whether caffeine could suppress alcohol-induced activation of this reward pathway to substantiate our previous results (3).
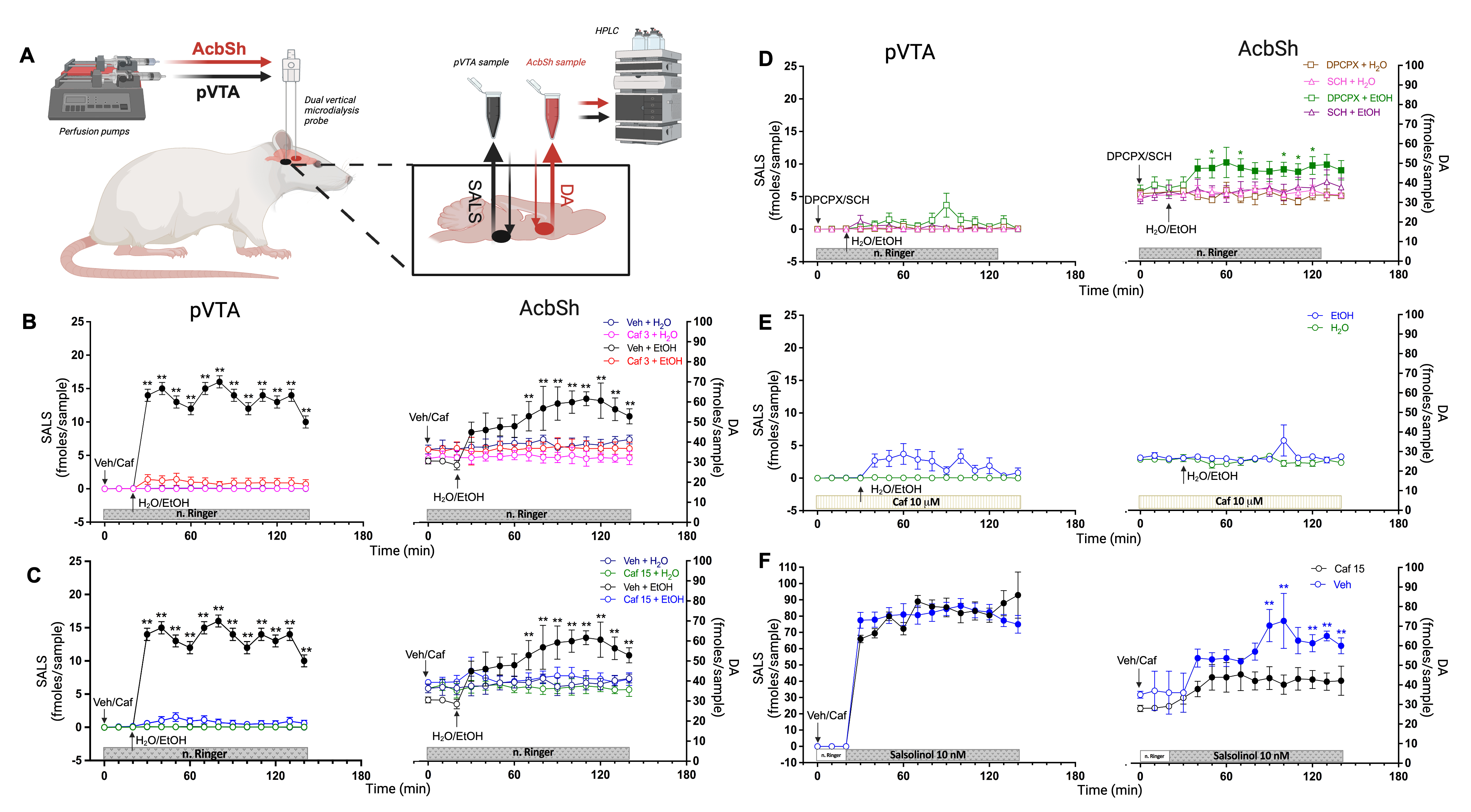
The Mesolimbic Pathway consists of dopaminergic neurons originating in the Ventral Tegmental Area (VTA) and projecting to the Nucleus Accumbens. Alcohol and other drugs of abuse enhance dopaminergic activity in the AcbSh (6). In 2021, we demonstrated that alcohol metabolism into salsolinol—formed via the condensation of dopamine with alcohol-derived acetaldehyde in the posterior VTA—plays a critical role in activating the Mesolimbic Pathway (7). Thus, our initial experiments assessed whether caffeine could prevent salsolinol formation in the VTA and the resulting increase in dopaminergic activity in the AcbSh. To simultaneously monitor salsolinol in the VTA and dopamine in the AcbSh, we implanted microdialysis probes in alcohol-naive, freely behaving rats and administered alcohol (1 g/kg) orally (Fig. 1 A). Remarkably, caffeine completely suppressed salsolinol formation in the VTA and the subsequent dopamine increase in the AcbSh (Fig. 1 A, B). As a nonselective antagonist of adenosine A1 and A2A receptors (8), caffeine’s effects were further explored using selective antagonists—DPCPX for A1 and SCH-58261 for A2A. The results highlighted A2A antagonism as the mechanism by which caffeine blocks salsolinol formation and dopaminergic activation (Fig. 1 D). Targeted perfusion of caffeine into the VTA also prevented these alcohol-induced changes, emphasizing the VTA as a critical site of caffeine action (Fig. 1 E).
Figure. 1
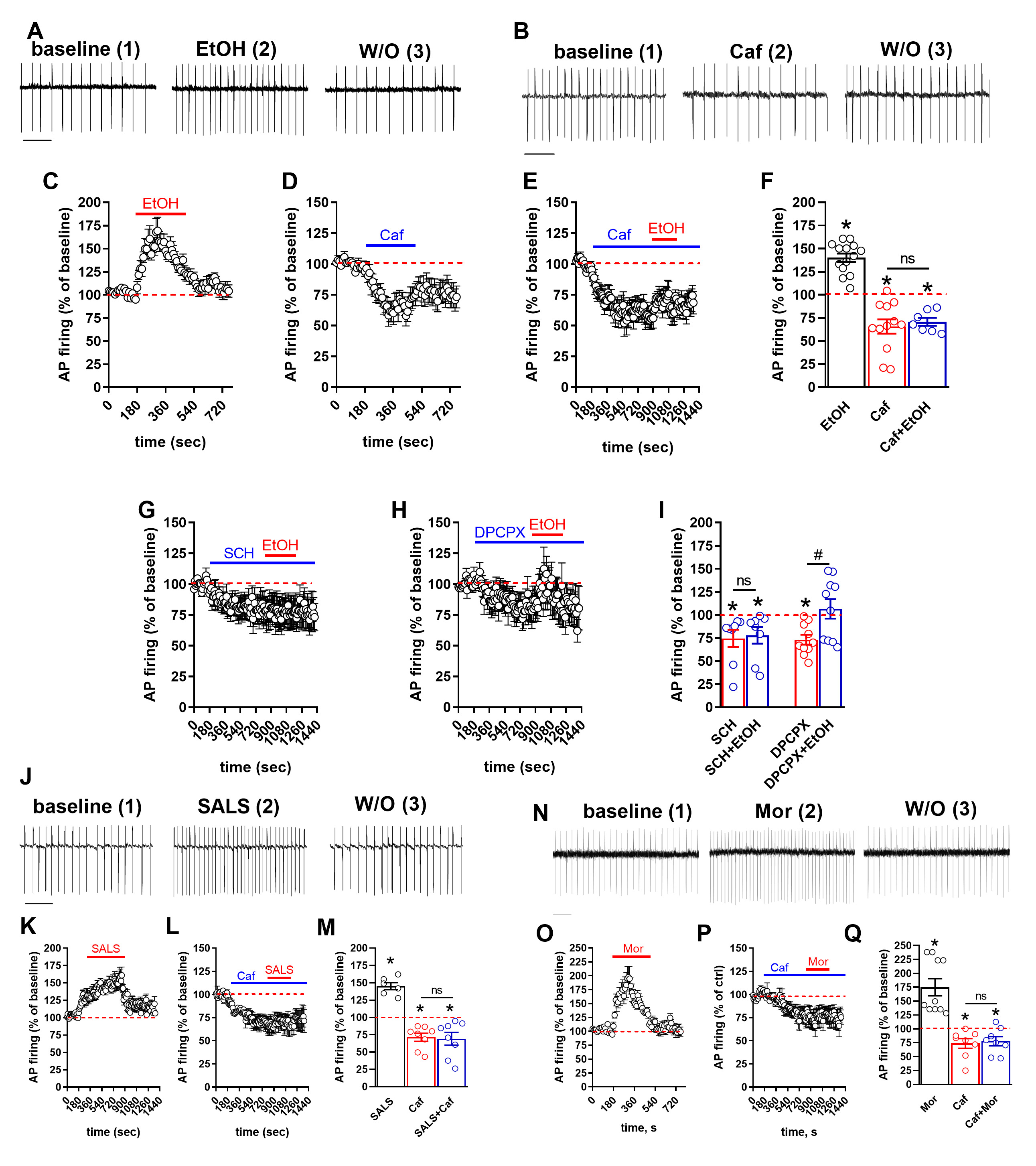
To validate these findings, we performed in vitro patch-clamp recordings of VTA dopaminergic neurons. Consistent with in vivo results, caffeine and the A2A adenosine receptor antagonist SCH-58261 prevented alcohol-induced firing of these neurons (Fig. 2 A-I). Surprisingly, caffeine also inhibited neuron firing induced by direct salsolinol or morphine exposure in vitro (Fig. 2 J-Q), indicating that caffeine’s effects extend beyond inhibiting salsolinol synthesis.
Figure. 2
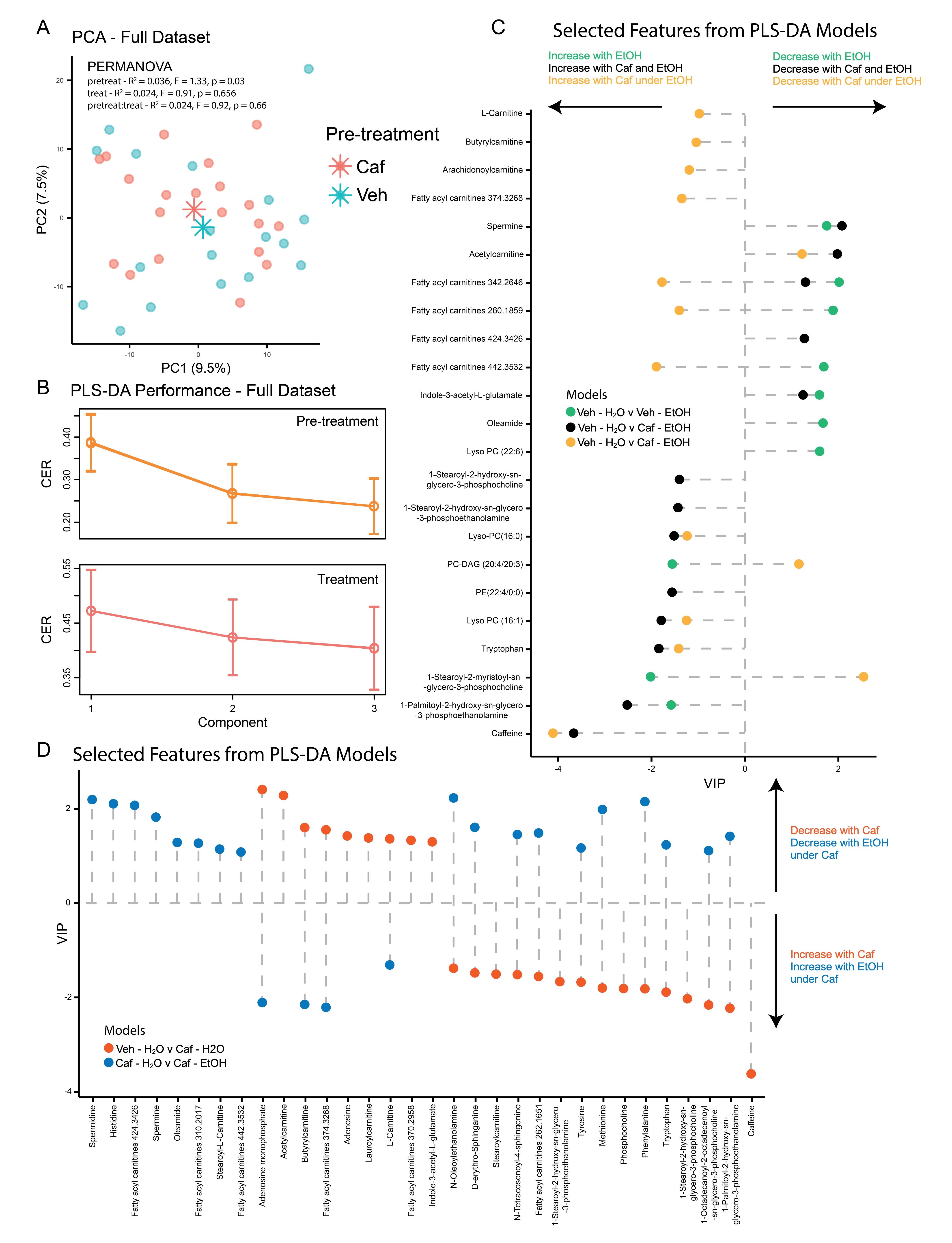
To uncover additional salsolinol-independent mechanisms, we conducted untargeted metabolomics analysis of VTA lysates from alcohol-treated rats, with and without caffeine pretreatment. Both alcohol and caffeine influenced lipid abundance, but caffeine counteracted alcohol-induced alterations in key lipid species (Fig.3). Brain lipids regulate processes such as signal transduction, synaptic plasticity, and neurotransmitter release, and their role in alcohol addiction is well-established (9-11). For example, caffeine normalized levels of lipids like oleamide, PC, and Lyso-PC, which are implicated in addiction (12-13), and resulted altered by alcohol in the present study (Fig.3 C). These findings suggest lipid regulation as an additional salsolinol-independent mechanism by which caffeine modulates the Mesolimbic Pathway.
Figure 3.
In conclusion, this study demonstrates for the first time that caffeine inhibits alcohol-induced activation of the mesolimbic dopaminergic pathway. Encouragingly, naltrexone, an FDA-approved drug for alcohol use disorder, mitigates alcohol’s reinforcing effects by targeting mesolimbic dopamine transmission (14). Hence, these results highlight the potential of caffeine, and particularly of A2A receptor antagonists, for developing preventive strategies for AUD.
Link to the study: https://www.nature.com/articles/s41398-024-03112-6
1. Mehta AJ. Alcoholism and critical illness: a review. World J Crit Care Med. 2016;5:27–35.
2. Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev. 1999;23:563–76.
3. Porru S, Maccioni R, Bassareo V, Peana AT, Salamone JD, Correa M, et al. Effects of caffeine on ethanol-elicited place preference, place aversion and ERK phosphorylation in CD-1 mice. J Psychopharmacol. 2020;34:1357–70.
4. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
5. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
6. Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–41.
7. Bassareo V, Frau R, Maccioni R, Caboni P, Manis C, Peana AT, et al. Ethanol-dependent synthesis of salsolinol in the posterior ventral tegmental area as key mechanism of ethanol’s action on mesolimbic dopamine. Front Neurosci. 2021;15:675061.
8. Daly JW, Shi D, Nikodijevic O, Jacobson KA. The role of adenosine receptors in the central action of caffeine. Pharmacopsychoecologia. 1994;7:201–13.
9. Shukla SD, Sun GY, Gibson Wood W, Savolainen MJ, Alling C, Hoek JB. Ethanol and lipid metabolic signaling. Alcohol Clin Exp Res. 2001;25:33S–9S.
10. Wang L, Li M, Bu Q, Li H, Xu W, Liu C, et al. Chronic alcohol causes alteration of lipidome profiling in brain. Toxicol Lett. 2019;313:19–29.
11. Bae M, Bandaru VV, Patel N, Haughey NJ. Ceramide metabolism analysis in a model of binge drinking reveals both neuroprotective and toxic effects of ethanol. J Neurochem. 2014;131:645–54.
12. Alen F, Decara J, Brunori G, You ZB, Buhler KM, Lopez-Moreno JA, et al. PPARalpha/CB1 receptor dual ligands as a novel therapy for alcohol use disorder: evaluation of a novel oleic acid conjugate in preclinical rat models. Biochem Pharmacol. 2018;157:235–43.
13. Philipsen MH, Phan NTN, Fletcher JS, Ewing AG. Interplay between cocaine, drug removal, and methylphenidate reversal on phospholipid alterations in Drosophila brain determined by imaging mass spectrometry. ACS Chem Neurosci. 2020;11:806–13.
14. Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–9.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in