Calcium and Calcium Carbonate Promote Biofilm Formation and Microbial Virulence
Published in Microbiology

Explore the Research
 sciencedirect.com
sciencedirect.com
ScienceDirect
About ScienceDirect Shopping cart Contact and supportTerms and conditionsPrivacy policy
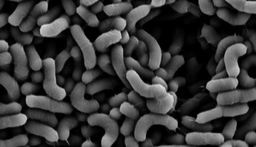
Microbial biofilms are of extreme clinical importance, as they are associated with many persistent and chronic infections (Costerton et al., 1999). The bacterium Pseudomonas aeruginosa is involved in chronic biofilm infections in compromised hosts, such as cystic fibrosis (CF) patients and those with burn wounds (Costerton et al., 1999). Similarly, the merging pathogen Mycobacterium abscessus causes infections of respiratory tract, skin and central nervous system in patients with CF and chronic obstructive pulmonary disease (Caimmi et al., 2018).
To date, the ability of biofilm-forming bacteria to generate complex architectures was exclusively attributed to their organic extracellular matrix (Stewart and Franklin, 2008), and primality to carbohydrate-rich polymers (i.e., extracellular polysaccharides or exopolysaccharides) (Branda et al., 2005). Various genetic analyses have provided strong evidence that biofilm exopolysaccharides play a fundamental structural role in biofilms of different bacterial species. P. aeruginosa isolates from lungs of cystic fibrosis (CF) overproduce the polysaccharide alginate, resulting in a mucoid colony phenotype and additional two exopolysaccharides, Pel and Psl, that play complementary roles in biofilms of P. aeruginosa (Colvin et al., 2012).
Notably, while organic matrices and their regulation differ between microorganisms (Steinberg and Kolodkin-Gal, 2015), the formation of the mineral calcium carbonate relies on simple building blocks, calcium and carbonate, generated from carbon dioxide. Thereof, targeting carbonate mineralization may provide an appealing general solution for biofilm infections (Keren-Paz and Kolodkin-Gal., 2020).
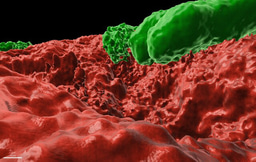
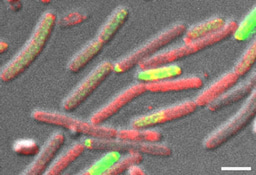
In this work (Cohen-Cymberknoh et al., In press), we discovered that the calcium-dependent 3D organization of the biofilm colony lead to transcriptional reprogramming of the bacterial community, and was essential for biofilm development. We could detect structured calcite in two unrelated lung pathogens – P. aeruginosa and M. abscessus. Using chemical inhibitors of key biomineralization enzymes or calcium uptake prevented biofilm formation and complex architecture by both pathogens. In addition, we were able to identify calcite in sputum samples taken from CF patients, suggesting that microbial mineralization is of clinical importance. Finally, in an ex vivo lung model, we tested the effects of inhibiting biomineralization on microbial virulence. Both chemical and genetic inhibition of calcium uptake and of carbonate accumulation blocked biofilm formation and lung colonization, and preventing damage inflicted by P. aeruginosa to lung tissues. For more information, please read our publication at https://www.sciencedirect.com/science/article/pii/S2589004222005041
Taken together, our results identify a previously overlooked process essential for bacterial biofilm development – the biologically regulated formation of mineral scaffolds. This role is not mutually exclusive to alternate roles of calcium as a signal, and as an agent capable of crosslinking alginate (Jacobs et al., In press). The conservation in calcium mineralization across the bacterial kingdom highlights the fundamental role it plays in biofilm biology, and could lead to novel therapeutic approaches for combating a broad range of persistent biofilm infections.
References:
Costerton, J.W., Stewart, P.S., and Greenberg, E.P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318-1322.
Caimmi, D., Martocq, N., Trioleyre, D., Guinet, C., Godreuil, S., Daniel, T., and Chiron, R. (2018). Positive Effect of Liposomal Amikacin for Inhalation on Mycobacterium abcessus in Cystic Fibrosis Patients. Open Forum Infect Dis 5, ofy034.
Stewart, P.S., and Franklin, M.J. (2008). Physiological heterogeneity in biofilms. Nat Rev Microbiol 6, 199-210.
Branda, S.S., Vik, S., Friedman, L., and Kolter, R. (2005). Biofilms: the matrix revisited. Trends Microbiol 13, 20-26.
Colvin, K.M., Irie, Y., Tart, C.S., Urbano, R., Whitney, J.C., Ryder, C., Howell, P.L., Wozniak, D.J., and Parsek, M.R. (2012). The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environmental microbiology 14, 1913-1928.
Keren-Paz, A., and Kolodkin-Gal, I. (2020). A brick in the wall: Discovering a novel mineral component of the biofilm extracellular matrix. N Biotechnol 56, 9-15. https://www.sciencedirect.com/science/article/pii/S1871678418318235
Cohen-Cymberknoh M., Kolodkin-Gal D., Keren-Paz A., Kapishnikov S., Green-Zelinger P., Shteinberg m., Zamir G., Kerem, E. and Kolodkin-Gal, I. (2022). Calcium carbonate mineralization is essential for biofilm formation and lung colonization. iScience, In press, https://www.sciencedirect.com/science/article/pii/S2589004222005041
Jacobs, H.M, O'neal., L., Lopatto, E., Wozniak, D.J, Bjarnsholt, T., and Parsek, M.R (2022). Mucoid Pseudomonas aeruginosa Can Produce Calcium-Gelled Biofilms Independent of the Matrix Components Psl and CdrA. J Bact., In press. https://journals.asm.org/doi/10.1128/jb.00568-21

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in