
Explore the Research
 sciencedirect.com
sciencedirect.com
ScienceDirect
About ScienceDirect Shopping cart Contact and supportTerms and conditionsPrivacy policy
We always aimed to resolve the molecular mechanisms that shape complex microbial communities, also designated biofilms.
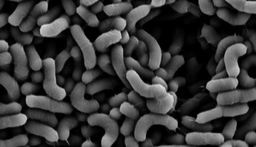
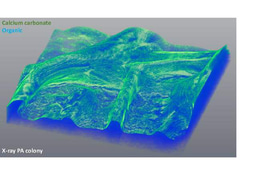
Our recent work demonstrates the intracellular calcium storage in a subpopulation of Bacillus subtilis cells: https://www.sciencedirect.com/science/article/pii/S258900422200579X. The genes that play a role in intracellular calcium homeostasis and carbonate production were all associated with complex morphologies, indicating that extracellular calcite scaffolds formed by Bacillus subtilis biofilms are most likely produced in an orchestrated manner. Our results imply that calcium carbonate is actively generated within a defined cell niche. It remains to be determined whether the intracellular deposits are of amorphous calcium carbonate. However, as phosphate is not enriched in these granules, the production of intracellular calcium carbonate by soil bacteria is a feasible hypothesis. Furthermore, we could detect the export of concentrated calcium to the extracellular mealie, where it was free to interact with the organic extracellular matrix.
Why is this important?
Of all examples of biomineralization, microbial-induced calcium carbonate precipitation (MICCP) is most frequently associated with microbial communities. Specifically, urea hydrolysis by urease-positive bacteria increases the local concentration of bicarbonate and elevates pH. When enough environmental calcium is present, those changes promote the spontaneous precipitation of calcium carbonate (Dhami et al., 2013). Recently, intracellular amorphous calcium carbonate (ACC) granules were detected in some species of Gram-negative autotrophic bacteria. Those intracellular deposits were suggested to contribute to photosynthesis (Blondeau et al., 2018) or chemolithoautotrophy https://www.nature.com/articles/s41396-020-00747-3, e.g., processes that contribute to single-cell survival.
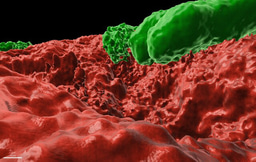
However, our previous discoveries of precisely organized mineral macrostructures (a "public good") within biofilms formed by a Gram-positive soil bacterium (npj Biofilms and Microbiomes) https://www.nature.com/articles/s41522-018-0051-8, https://www.nature.com/articles/npjbiofilms201531. Now we could demonstrate that the formation of this mineral is controlled by cellular development (https://www.sciencedirect.com/science/article/pii/S258900422200579X)
Collectively, these works suggest that biofilm biomineralization is tightly regulated, and calcium carbonate scaffolds contribute to biofilm colonies’ fitness. Our results indicated that Bacillus subtilis could produce calcium carbonate in neutral pH as it can alter the local pH within the territory. In addition, we could detect minerals in floating biofilms and across different media. The mineral structure acted as a structural scaffold supporting the 3D architecture of the colony.
Therefore, our reports collectively challenge the current view of biofilm development as a process depending solely on organic ECM production. They raise several unanswered questions regarding the developmental role of mineral scaffold formation within a bacterial community. Is mineralization a controlled process with importance to biofilm development and function? What is the role of mineral structure in the development of a differentiated biofilm community? For example, the limitation of nutrient flow and the exclusion of other cations may have critical roles in gene expression. Finally, the fitness advantages that regulated mineralization confers to the community remain to be determined. For more details, read our paper.
Additional References:
Dhami, N.K., Reddy, M.S., and Mukherjee, A. (2013). Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. J Microbiol Biotechnol 23, 707-714.
Blondeau, M., Sachse, M., Boulogne, C., Gillet, C., Guigner, J.M., Skouri-Panet, F., Poinsot, M., Ferard, C., Miot, J., and Benzerara, K. (2018). Amorphous Calcium Carbonate Granules Form Within an Intracellular Compartment in Calcifying Cyanobacteria. Frontiers in Microbiology 9.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in