Calcium Chronicles: Viral Rhodopsins and the Unseen Threads of Cellular Control
Published in Microbiology, Protocols & Methods, and Cell & Molecular Biology

Here I am, flipping through my old labbook, attempting to embody my former self as I recall the behind-the-scenes of our recently published work.
Shortly put, we demonstrated that certain viral channelrhodopsins - ion channels activated by light from giant viruses - reside intracellularly and can release calcium upon illumination. This triggers a calcium signal that wields control over cellular and animal behavior.
It feels bitter to squeeze so much work into a few lines, but I want to make sure this post does not become a paper rehash. Instead, I want to share some personal stories and events that framed our thought process, as candidly as possible, hoping to contextualize how this work came to be.
The beginning of it all
Optogenetics, the method of manipulating cell or animal behaviour using light and light-responsive proteins - like rhodopsins -, isn't just the stuff of sci-fi movies. While the image of a mouse with an optic fiber embedded in its brain might sound like a scene from a futuristic saga, optogenetics has already proven its value, even leading to the partial visual recovery of a human patient.
Amidst the optogenetics hype, we get involved in a project to characterize rhodopsins with novel functions, aiming to expand the optogenetic toolkit.
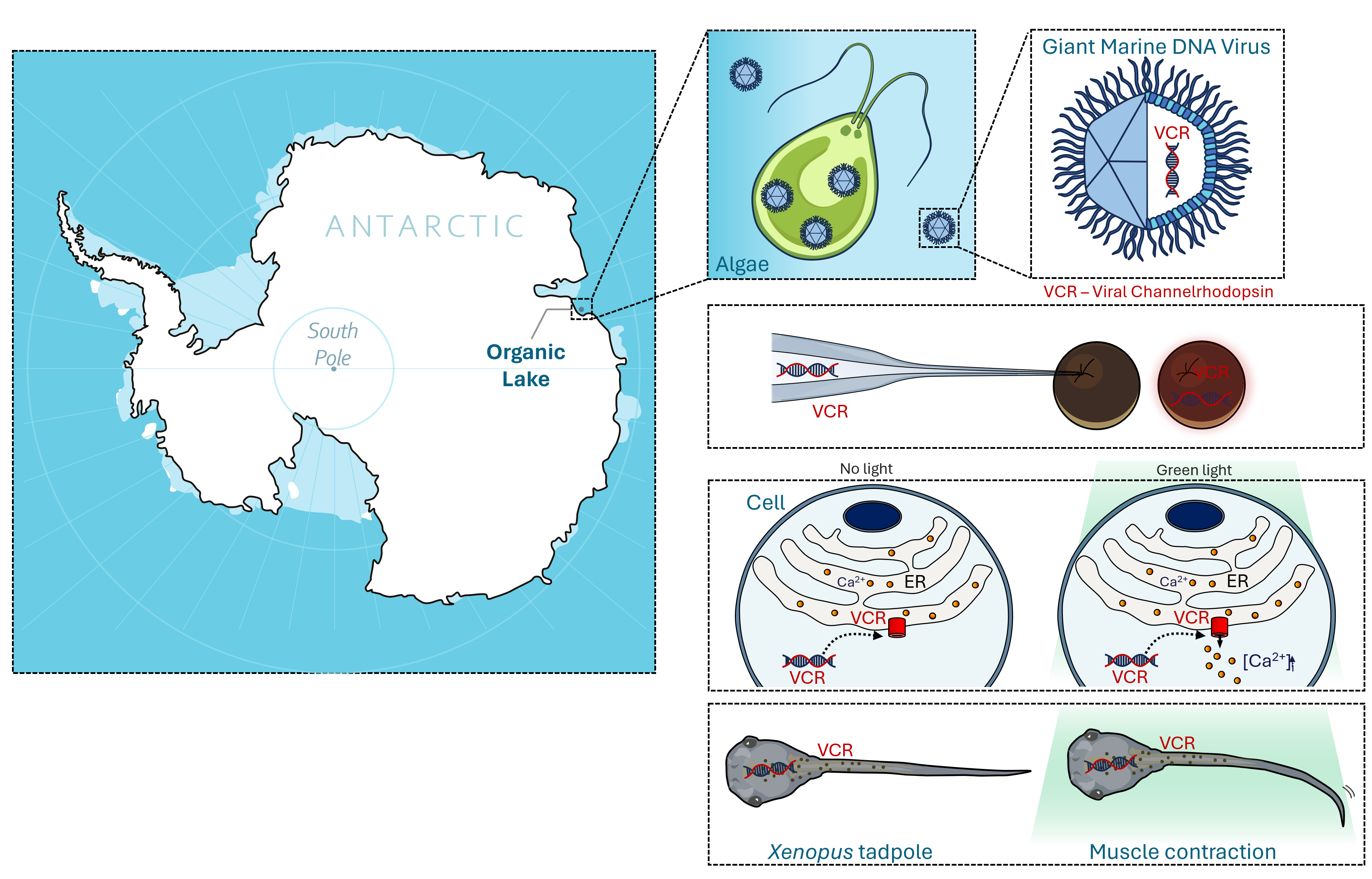
We targeted giant marine viruses. These viruses are bigger than some bacteria and only 45 times smaller than their host. Picture this…if you were a giant virus your host would be the size of an African Bush Elephant. Their accompanying large genomes code for an extensive catalogue of proteins, including rhodopsins. Their sequences deviate so much from other known rhodopsins that we expected them to have unique and potentially exploitable functions.
Pretty quickly after starting the project, Dmitrii cracked the structure of OLPVR1, a channelrhodopsin from a virus found in the Antarctic. In parallel, Alexey demonstrated that another viral rhodopsin, VirChR1, acted as a cation channel at the surface of neuroblastoma cells. While we suspected OLPVR1 operated similarly, it remained unrecorded at this point.
A letter to the victims of pre-conceived bias
The next steps seemed straightforward. Express the OLPVR1 gene, in our case in Xenopus oocytes, and characterize the light-activated currents at the membrane.
Fast forward two months of late nights in the lab, and there I was, sitting in front of the electrophysiology set-up, yet still no sign of OLPVR1 function. Imagine… a darkened lab only illuminated by the glow of firefly-like amplifier LEDs. In the background, the soporific hum of the equipment and the occasional sharp crinkling sound of aluminium foil. Yes, at this point, I had wrapped the whole set-up in aluminium – my desperate attempt to insulate it, convinced that reducing the noise would expose some picoscopic flicker of channel activity…It did not.
I guess I had no option left but to start questioning everything, which in retrospect I should have done sooner. Eventually, I stripped down my protocol, shifting from trying to record cationic currents to unassumingly record any type of currents. That's when I saw it...
Xenopus Appreciation Day
Looking at the current traces, I had a feeling of déjà vu – a fitting feeling in a French lab. These currents had an uncommon profile. After skimming through a stack of research papers, we found the traces we sought: calcium-activated chloride currents (CaCCs), intrinsic to our expression system - Xenopus oocytes. Except that they were now seemingly activated by light. The result was so unexpected given what was known, we had spent months searching for membrane cation channels, using conditions meant to block these endogenous chloride channels.

So, let’s take a moment to appreciate this wonderful model, the Xenopus oocytes. Before so popular and now often left on the sidetracks like a retired racing hound. It is the reason why we were able to capture the calcium-release mediated by OLPVR1. Had we used any other model, lacking calcium-activated channels, we would have missed it completely.
What is even a floppy disk?
Because there were no other currents at the membrane besides endogenous calcium-activated currents we wondered if OLPVR1 was even expressed at the surface of the oocytes.
When working with oocytes, one often hears tales of endlessly difficult quests to localize proteins - a prospect that appals even those well-versed in this model. There was, however, a very old luminometer in the lab that had been used for it.
A sepia envelope was just next to it. I still remember the confused look on Mathilde’s face when I opened the envelope and slid out a vintage-looking piece of electronics – the old floppy disk. Almost telepathically, we agreed there were probably fresher ways to accomplish what we needed.
We found the answer in a Promega Luminescence system called NanoGlo. After several rounds of optimization we established a fast, simple and reproducible method to quantify and localize proteins in Xenopus oocytes, craftily baptized by Michel as XenoGlo. Spoiler alert: article coming soon…
With this technique, we demonstrated OLPVR1 was exclusively expressed intracellularly.
The fruits of scientific exchange: a shiny new tool
New ideas often hinge on unplanned encounters. Michel was on the LabEX ICST meeting presenting the data we had so far, when he crossed paths with François Rassendren. Having used for his own research a calcium fluorescence sensor fused to his protein of interest, he suggested we did the same. It was this offhand comment, together with some clever experimental conditions, that eventually allowed us to get the strongest piece of evidence that OLPVR1 was permeating calcium.

We had now in our hands a potential optogenetic tool to photocontrol calcium-release from intracellular stores.
Social media gems
There we were, bouncing ideas on our weekly 10-min meeting with the lingering aroma of coffee, when a post pops up on social media: a picture of a Xenopus tadpole with the #University of Bordeaux.
The idea of using tadpoles to proof-of concept the use of OLPVR1 for optogenetics had already been birthed before. But after attempting to obtain this model from two international providers that refused our request, this idea had died down. However, following that chirp of what was then Twitter, we eventually established a protocol with Pierre Thibaud, Sandrine and Nadine in Bordeaux.
The first time we saw it we were in disbelief. I remember Guillaume enthusiastically shining light on the tadpoles in a pattern reminiscent of a 70s disco night. Ultimately, we demonstrated that OLPVR1 expressed in tadpoles enabled the control of their movements with light.
A room full of electrophysiologists
Electrophysiologists are a special breed of researchers. How could they not be? We start teaching students about bioelectricity by showing them Luigi Galvani casually hanging frog legs on his fence. Only those with an inclination for the esoteric and an unhealthy obsession with toxins end up in electrophysiology.
My point being, there is nothing quite like a room full of electrophysiologists. Sporting double-ended crocodile-clip cables like a stethoscope, ready to ground any electrical noise that comes interrupting. The atmosphere is electric as ideas spark and flicker (puns intended) and old-school electrophysiology secrets are exchanged.
It was in this context, that I see Guillaume’s eyes glisten, while he drops the story behind curare to Amandine as naturally as a gossip between neighbours. That was the trigger for our last experiments. We used curare to block neuron-muscle communication and demonstrate that light-induced OLPVR1 calcium release in muscle cells is sufficient to incite contraction.
Why Would Anyone Care?
I have all flavours of reasons to give you.
If you are into membrane proteins, viral channelrhodopsins are incredibly interesting. They are small proteins with no retention signals that somehow still reside exclusively in internal membranes. They must hold uncharted information on preferential protein localization.
Rhodopsin fanatics are also in for a treat. These are the first microbial rhodopsins shown to have a biological role in internal membranes. It might be something worth considering if you are struggling to characterize your rhodopsin of interest.
From the micro/ecological perspective, giant marine viruses own a pivotal role in world ecology and climate. They prey on phytoplankton – major primary producers -, controlling their population dynamics and ultimately carbon and nutrient cycles. Viral rhodopsins have the hallmarks to be the main players in how giant viruses fulfil their role. They constitute a direct link between the hosts fundamental energy source to fix carbon – light – and connects it to the most ubiquitous cellular control signal – calcium. Are giant viruses the true puppeteers of carbon cycles?
Staying within the project's original goal, viral rhodopsins can enable the photocontrol of intracellular calcium release in both cells and animals. By leveraging the spatial-temporal resolution of light one could use these proteins to answer relevant questions in a broad range of fields as well as devise optogenetic-based therapies. Could viral rhodopsins acting directly on muscle cells offer a therapeutic advantage in neuromuscular disorders?
To finish, I propose a quick mental exercise. Create a mind map of calcium and whatever cellular process you can think of. Very likely, you will need no more than 3 nodes to connect them both. Calcium is such a ubiquitous signalling molecule, it participates in most cellular functions, being physiological or pathological. Not all circumstances will necessarily benefit from the time and space precision light and OLPVR1 can offer, but some might. So besides what we ourselves can envision for future avenues of research, we are curious to see what you, and the broader public of Nature Communications, are able to conceive from our findings.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in