Can malaria vaccines exploit complement for enhanced efficacy?
Published in Microbiology

Developing highly efficacious vaccines for human malaria caused by Plasmodium falciparum is a major global health priority, although this has proven to be immensely challenging over the decades. Significant progress towards effective malaria vaccines has been achieved in recent years, most notably with the demonstrated efficacy of the RTS,S vaccine in phase 3 trials. However, overall vaccine efficacy is modest in children (around 50% in the first year) and wanes relatively quickly. Other malaria vaccines have either demonstrated little or no efficacy in clinical trials, or failed to achieve higher efficacy or greater durability than RTS,S in malaria endemic populations. This raises the obvious question of what strategies might be used to increase vaccine efficacy. One strategy might be to maximize interactions between vaccine-induced antibodies and complement.
In recent studies we have demonstrated that naturally acquired antibodies that target merozoites (the form parasites that infect red blood cells during blood-stage parasite replication) can fix and activate complement to inhibit invasion of red blood cells and prevent blood stage replication. Additionally, we established that antibodies to sporozoites of the right type and target specificity can also effectively fix and activate complement to inhibit sporozoite motility and host-cell invasion and cause sporozoite cell death. We have shown that the RTS,S vaccine and vaccines based on merozoite surface proteins can induce these functional antibodies. Furthermore in studies of naturally-acquired immunity, complement-fixing antibodies were more strongly associated with protective immunity that other antibody measures.
Malaria vaccines targeting merozoites and sporozoites primarily mediate immunity through the induction of antibodies. While these antibodies can directly block host-cell invasion, this effect typically requires high antibody concentrations. In the presence of complement, antibody inhibition can be increased if the antibodies have good complement fixing activity. This effect has also been reported with some viruses, such as influenza, West Nile virus, and hepatitis C.
Given the challenge of achieving high protective efficacy by malaria vaccines, investigating vaccine strategies that can induce potent complement fixing antibodies may reveal new solutions. Achieving strong antibody-complement interactions could be achieved by refocussing the vaccine response on specific epitopes through vaccine design, and enhancing induction of IgG3 antibodies through specific adjuvants or delivery platforms. These concepts are reviewed in detailed in the recent paper by Kurtovic et al:
https://onlinelibrary.wiley.com/doi/full/10.1111/imr.12802
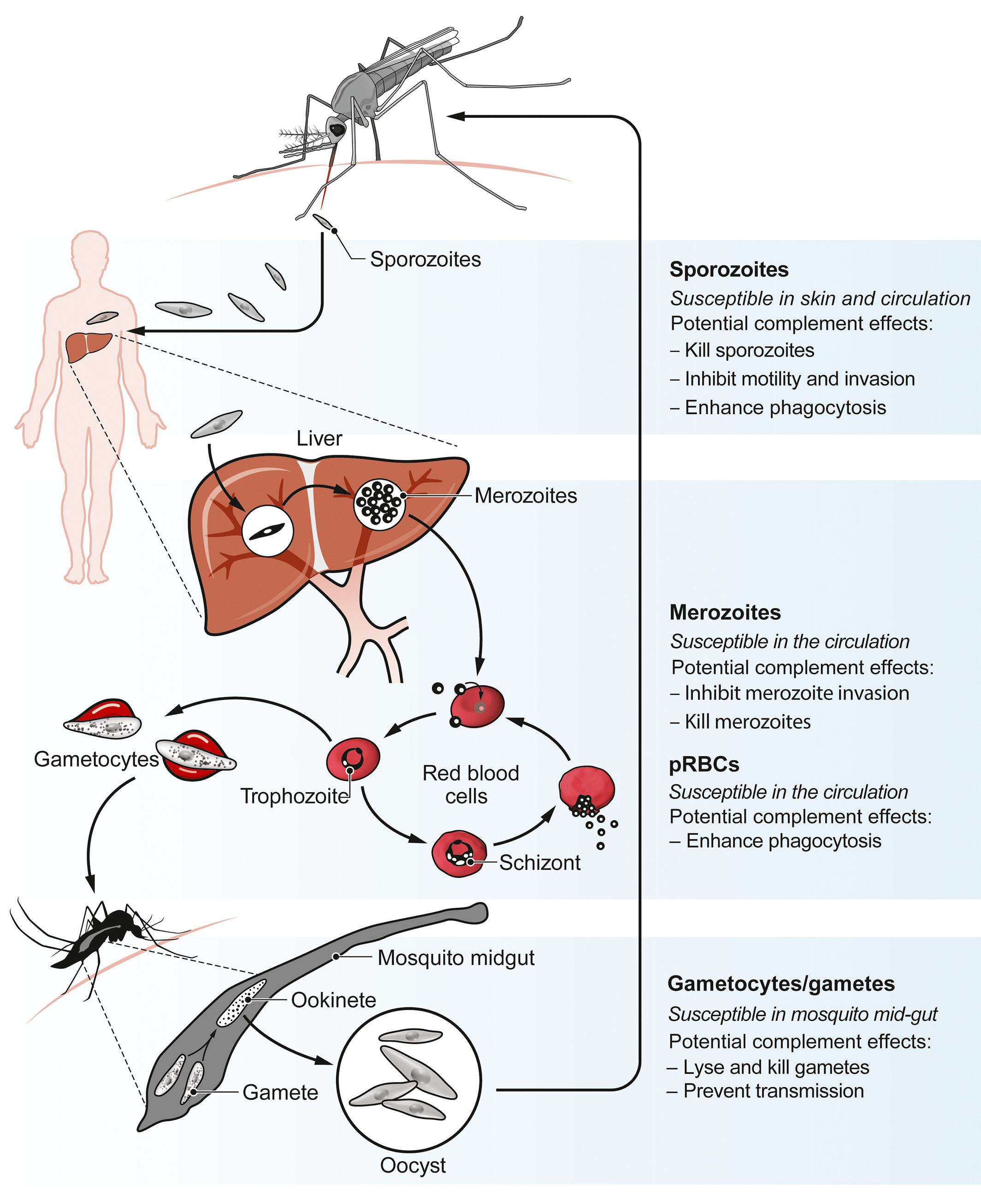
Figure: Plasmodium life cycle and opportunities for antibody‐complement attack. (Image taken from Kurtovic L, et al, Immunological Reviews 2019 and Beeson JG et al, Science Translational Medicine 2019)


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in