Cardiovascular Biomechanics Through the Lens of Artificial Intelligence
Published in Computational Sciences, General & Internal Medicine, and Public Health
My journey into cardiovascular biomechanics began with a fascination for the heart’s powerhouse—the left ventricle (1). When I arrived in the United States in 2003, I was captivated by the intricacies of the heart in motion, not just metaphorically but literally. I immersed myself in advanced imaging techniques like speckle tracking and tissue Doppler echocardiography, which revealed the elegant choreography of myocardial contraction and relaxation in real-time. These visual insights weren’t just beautiful—they offered a window into how the disease takes hold, setting the stage for a translational research path that could bridge clinical imaging with the biological underpinnings of cardiac dysfunction.
As my work evolved, so did my questions. I explored how the heart’s deformation patterns shift in the face of disease, mapping these changes across a spectrum of cardiovascular conditions. A defining moment came during a study of transplanted hearts. We connected mechanical behavior to gene expression profiles there, opening an experience that transformed my research trajectory (2). I dove into big data, leveraging tools like heatmaps and advanced models to probe the heart’s response to systemic diseases—not just through images, but through patterns, signatures, and networks.
The Birth of AI in Echocardiography
A shift toward data-intensive research brought me into collaboration with data scientists at the Icahn School of Medicine at Mount Sinai. Together, we became one of the earliest groups to apply artificial intelligence (AI) to echocardiographic data (3, 4). What began as an exploratory partnership soon turned into a robust research pipeline. Building on early breakthroughs using echocardiography-derived tabular data, I started exploring more complex cross-modality questions—Could we predict the heart's mechanical function—how it beats and moves—using only the electrical signals from an electrocardiogram (ECG)?
To our excitement, the answer was yes.
We could use AI algorithms to accurately predict key echocardiographic features such as diastolic function from surface ECGs (5). These early results were soon followed by teams at other institutions applying AI to ECG signals to predict ejection fraction—the world had caught on to the idea.
Building Innovation
I undertook a new challenge—establishing a cutting-edge cardiology program at West Virginia University. I founded an Innovation Center focused on integrating wearable technology, digital health tools, and advanced imaging techniques—from point-of-care ultrasound to cardiac MRI—to better predict cardiovascular outcomes.
A pivotal moment came when we secured a National Science Foundation grant titled Bridges to Digital Health, which paired clinical teams with engineers to develop real-world digital solutions (6). It was here that I met Dr. Naveena Yanamala, a rising star in biomedical data science. Her expertise in AI and translational research made her an ideal collaborator. Together, we began to build a vibrant ecosystem of clinicians, biomedical engineers, and data scientists focused on developing next-generation solutions in cardiovascular diagnostics (7,8).
The Grand Challenge: Generating Synthetic Echocardiograms from ECGs
Eventually, as the team returned to the East Coast, joining Rutgers Robert Wood Johnson Medical School, one audacious idea captured our imagination: What if we could recreate the entire mechanical motion of the heart—from a simple electrocardiogram?
This wasn’t just theory. Electrical activity in the heart influences the surrounding electric field—a signal rich with biomechanical information. If decoded properly using generative AI, we believed it was possible to reconstruct a visual representation of a beating heart, complete with tissue motion and chamber geometry.
Together with Dr. Yanamala’s team and an exceptional graduate student, Aditya Radhakrishnan, we set out to develop a generative adversarial network (GAN) capable of creating synthetic tissue Doppler echocardiography waveforms from paired ECG data. This required meticulous synchronization: we only used ECG and echo images recorded within an hour of each other to ensure accurate mechanical-electrical correlation.
Cracking the Code of Waveform Generation
One of our toughest challenges was translating spectral Doppler signals into clean line waveforms that could be used for training the GAN. The spectral images contain overlapping velocity signals, and interpreting the "envelope"—the outer edge of the waveform—proved as tricky for algorithms as it often is for humans.
We developed a standardized method to extract consistent envelopes, reducing variability and improving model accuracy. The results were stunning: synthetic waveforms were nearly indistinguishable from real tissue Doppler images.
To validate this, we conducted a randomized Turing test. Board-certified echocardiographers were shown a mix of real and synthetic waveforms and asked to identify which was which. Most could not tell the difference. Furthermore, the synthetic signals demonstrated strong physiological correlations and could even predict diseases.
From Concept to Clinical Impact
We tested the model on an independent South American dataset that included ECGs and long-term clinical outcomes. Our synthetic echocardiograms—generated purely from ECGs—predicted all-cause mortality, even after adjusting for age, gender, and abnormal heart rhythms.
Today, this algorithm is patent-pending, and we’re continuing to refine its capabilities.
The Future: Population Screening, Personalized Care, and Beyond
What excites me most is the potential impact on public health. The speed of longitudinal motion and strain—the subtle deformation of the heart muscle—often changes well before a measurable drop in ejection fraction. These early biomechanical shifts are crucial in detecting subclinical disease, allowing us to intervene before structural damage occurs.
Our technology could usher in a new era of precision screening, especially in at-risk populations. They may also have the potential to monitor therapeutic interventions—such as patients on cardiotoxic chemotherapy or those undergoing novel treatments for heart failure. Instead of arbitrary testing intervals, we could implement physiology-driven monitoring based on real-time biomechanical changes.
Even more importantly, the interpretability of waveform-based AI outputs allows clinicians to trust and adopt these tools better. Seeing a synthetic but physiologically accurate heartbeat generated from a simple ECG could bridge the gap between black-box AI and human expertise.
We stand on the brink of a transformative era in cardiovascular biomechanics. By merging data science with clinical insight, we’re not just predicting disease—we’re reimagining how we see the beating heart.
Let’s step into this future, riding the wave of every synthetic heartbeat—where science and discovery heal.
References:
- Sengupta PP, Korinek J, Belohlavek M, Narula J, Vannan MA, Jahangir A, Khandheria BK. Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol. 2006 Nov 21;48(10):1988-2001. doi: 10.1016/j.jacc.2006.08.030. Epub 2006 Oct 31. PMID: 17112989.
- Eleid MF, Caracciolo G, Cho EJ, Scott RL, Steidley DE, Wilansky S, Arabia FA, Khandheria BK, Sengupta PP. Natural history of left ventricular mechanics in transplanted hearts: relationships with clinical variables and genetic expression profiles of allograft rejection. JACC Cardiovasc Imaging. 2010 Oct;3(10):989-1000. doi: 10.1016/j.jcmg.2010.07.009. PMID: 20947044.
- Sengupta PP, Huang YM, Bansal M, Ashrafi A, Fisher M, Shameer K, Gall W, Dudley JT. Cognitive Machine-Learning Algorithm for Cardiac Imaging: A Pilot Study for Differentiating Constrictive Pericarditis From Restrictive Cardiomyopathy. Circ Cardiovasc Imaging. 2016 Jun;9(6):e004330. doi: 10.1161/CIRCIMAGING.115.004330. PMID: 27266599; PMCID: PMC5321667.
- Narula S, Shameer K, Salem Omar AM, Dudley JT, Sengupta PP. Machine-Learning Algorithms to Automate Morphological and Functional Assessments in 2D Echocardiography. J Am Coll Cardiol. 2016 Nov 29;68(21):2287-2295. doi: 10.1016/j.jacc.2016.08.062. PMID: 27884247.
- Sengupta PP, Kulkarni H, Narula J. Prediction of Abnormal Myocardial Relaxation From Signal Processed Surface ECG. J Am Coll Cardiol. 2018 Apr 17;71(15):1650-1660. doi: 10.1016/j.jacc.2018.02.024. PMID: 29650121.
- Sengupta PP, Adjeroh DA. AI tracks a beating heart's function over time. Nature. 2020 Apr;580(7802):192-194. doi: 10.1038/d41586-020-00819-6. PMID: 32214236.
- Yanamala N, Krishna NH, Hathaway QA, Radhakrishnan A, Sunkara S, Patel H, Farjo P, Patel B, Sengupta PP. A vital sign-based prediction algorithm for differentiating COVID-19 versus seasonal influenza in hospitalized patients. NPJ Digit Med. 2021 Jun 4;4(1):95. doi: 10.1038/s41746-021-00467-8. PMID: 34088961; PMCID: PMC8178379.
- Hathaway QA, Yanamala N, Siva NK, Adjeroh DA, Hollander JM, Sengupta PP. Ultrasonic Texture Features for Assessing Cardiac Remodeling and Dysfunction. J Am Coll Cardiol. 2022 Dec 6;80(23):2187-2201. doi: 10.1016/j.jacc.2022.09.036. PMID: 36456049.
Follow the Topic
-
Nature Cardiovascular Research

This journal aims to serve a multidisciplinary community by providing a unifying publishing platform that will champion and disseminate original and important advances in basic, translational, clinical and public health research in cardiovascular biology and haematology.
Your space to connect: The Nitric oxide signalling in cardiovascular health and disease Hub
A new Communities’ space to connect, collaborate, and explore research on Cardiovascular Physiology, Clinical Medicine, and Diseases!
Continue reading announcement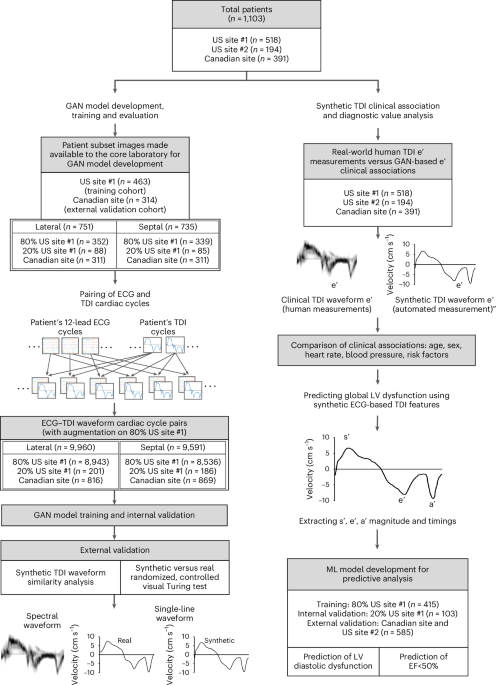





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in