Cascade Photochemical Reactions with Carbon Nitrides
Published in Chemistry

Synthesis of benzylidenebenzylamine (or simply imine) from two benzylamine molecules is probably one of the most studied reactions that is used by many research groups in the world to check if a newly prepared material can act as the photocatalyst. It requires a sacrificial oxidant, which typically is oxygen. A control experiment in this reaction is conducted under anaerobic conditions. Conversion of benzylamine and yield of the imine are expected to be zero.
In 2018, my group has been working on poly(heptazine imide), a type of graphitic carbon nitride, which we also tested in this reaction. It was surprising to find ~15% conversion of benzylamine under anaerobic conditions.
At that time my group already had experience in photocharging of poly(heptazine imide) with electrons by using sacrificial reductants and light. Therefore, we assumed that in the reaction with benzylamine, poly(heptazine imide) acts not only as a catalyst, but also as a stoichiometric oxidant. In other words, electrons, protons and ammonium cations, which are released in the reaction are stored in the material.
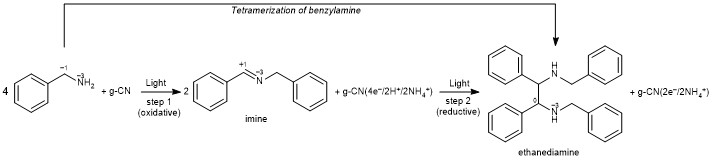
Therefore, carbon nitride is reduced. To check this idea, we set a couple of experiments using a variable mass of carbon nitride. As I expected, the yield scaled with the mass of the material. However, not linearly as I expected. For example, in a typical experiment to reach complete conversion of benzylamine (0.1 mmol) at least 160 mg of carbon nitride was necessary. Of course, in such a case, we cannot talk about “catalysis”, because the amount of carbon nitride exceeds the amount of benzylamine. A more appropriate term is a “photochemical reaction” between benzylamine and carbon nitride. More intriguing is that in these experiments in addition to the imine, a certain amount of ethanediamine was also formed.
This photochemical reaction is a cascade process. In the first step, 4 benzylamine molecules are converted into 2 imine molecules, while 4 electrons, 2 protons and 2 ammonium cations are stored in the carbon nitride. In the second step, 2 electrons and 2 protons are combined with 2 imine molecules into one ethanediamine molecule, while 2 electrons and 2 ammonium cations remain stored in the carbon nitride.
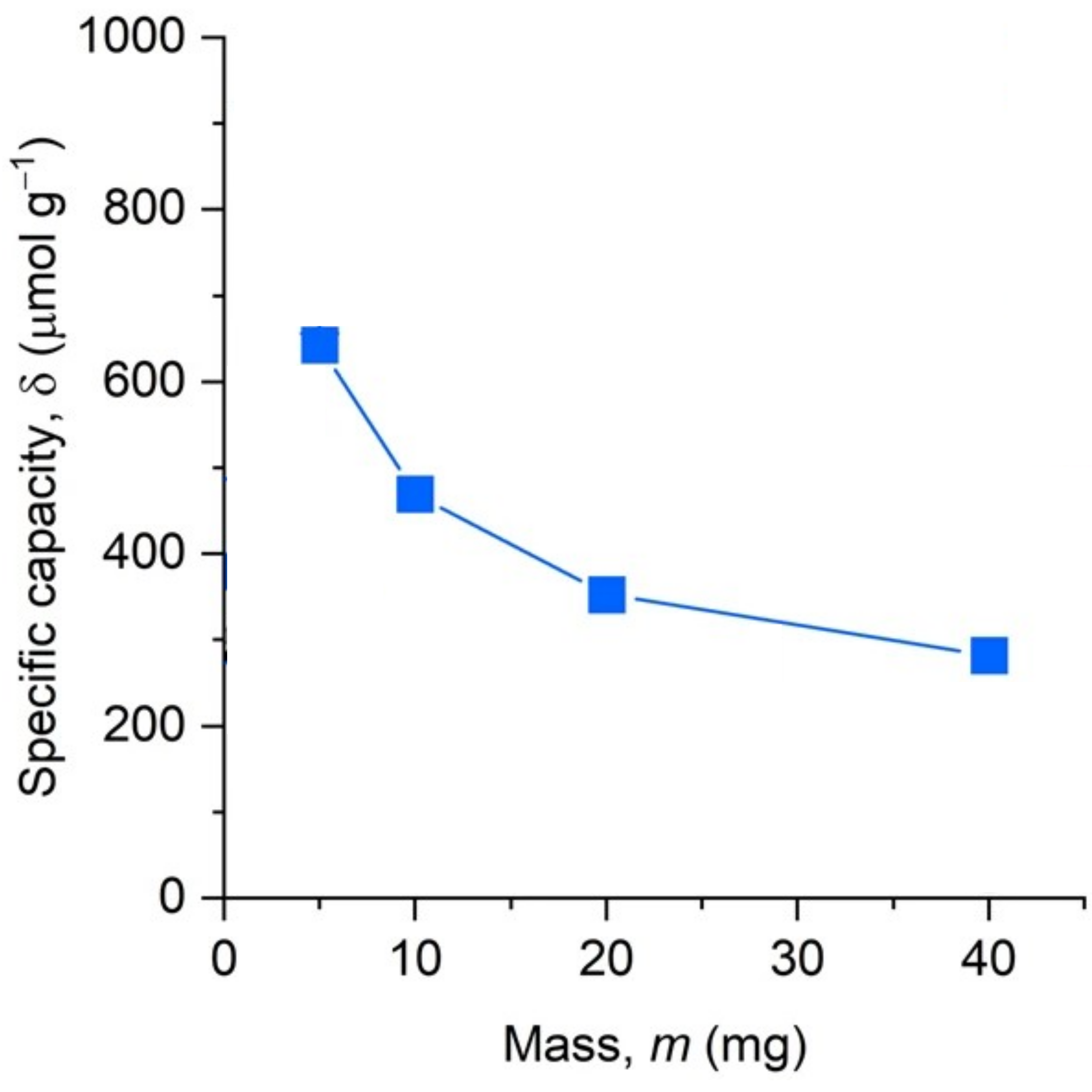
However, why conversion of benzylamine and yield of ethanediamine did not scale linearly with the mass of carbon nitride? We set a series of experiments to answer this question. Namely, we measured the number of electrons stored in carbon nitride, structure g-CN(2e–/2NH4+) in the reaction scheme, depending on the mass of the material that we added into the reaction mixture and irradiated with light. When we normalized the measured number by the mass of carbon nitride, it was exciting to find that this number is not constant. The greater mass of carbon nitride, the less electrons it stores. In other words, a greater benzylamine-to-carbon nitride ratio favours storage of a greater number of electrons in the material.
Conversion of imine into ethanediamine involves hydrogen atom transfer from the photocharged g-CN, structure g-CN(4e–/2H+/2NH4+), to the imine. Therefore, it is essential to evaluate the energy of hydrogen atom abstraction from this species, a parameter similar to the bond dissociation energy in small molecules. With this question I approached Prof. Thomas Kühne, with whom we collaborated on one project before. His team modelled sodium poly(heptazine imide) loaded with electrons and ammonium cations. The results of modelling revealed that the energy of hydrogen atom abstraction from sodium poly(heptazine imide) is not constant. But it depends on the extent to which this carbon nitride material is hydrogenated. For example, when one hydrogen atom is stored over 2 heptazines, abstraction of hydrogen atom is 72 kJ mol-1 endothermic. This amount of energy is required to remove hydrogen atom from the material. On the other hand, when a hydrogen atom is stored over 20 heptazines, the energy decreases to 41 kJ mol-1 - it is easier to remove hydrogen atom from a material hydrogenated to lesser extent.
.gif)
The results of theoretical modelling confirmed that strongly reduced carbon nitrides are reluctant to give their hydrogen atoms to oxidants. From a practical standpoint, to avoid trapping hydrogen atoms in carbon nitride, a larger amount of the material per mole of reagent is required. This is an important aspect to consider when designing photochemical reactions with heterogeneous carbon nitride materials. However, stabilization of electrons in strongly-reduced carbon nitride allows preparing persistent carbon nitride radicals - compounds, which are stable in air.
A bonus point of this study is poly(heptazine imide) photocharged to the extent that the radical species survive in air for ~20 minutes - an impressive result for organic radical! It is formed by using ammonium formate – strong electron donor and a source of charge compensating ammonium cations, which remarkably well stabilize electrons in poly(heptazine imide).
Read more about cascade photochemical reactions and photocharging of carbon nitrides in our article in Nature Communications!
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in