Catalytic ammonia oxidation via chemical looping
Published in Chemistry
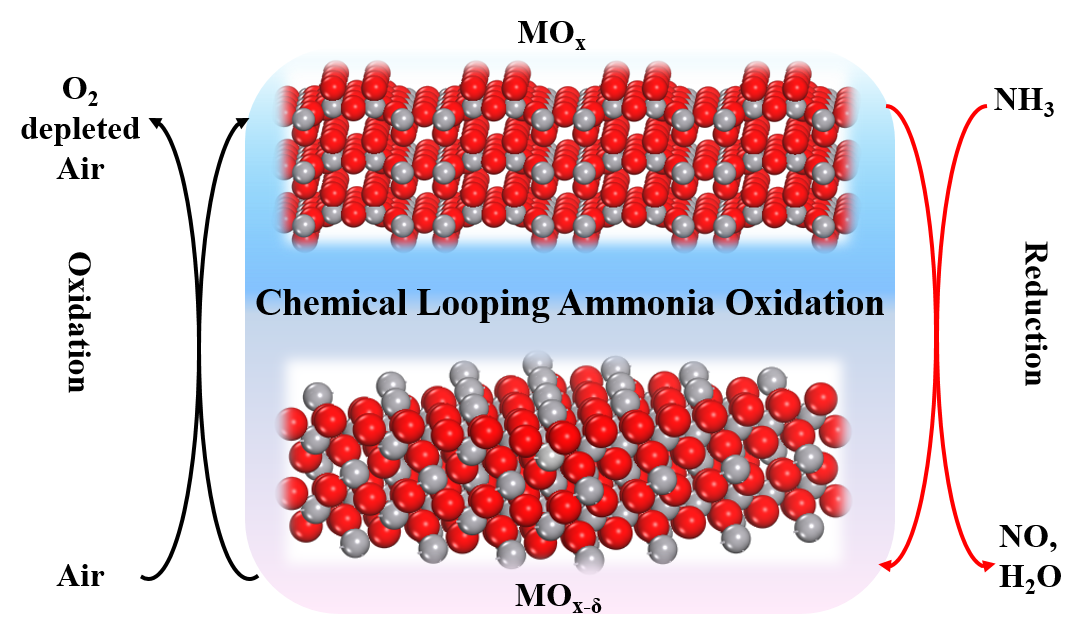
Catalytic oxidation of ammonia to NO is the core reaction for producing nitric acid, one of the top-ten bulk chemicals by annual consumption (over 100 million tons per year) and widely applied in the manufacturing of fertilizers, nylon, dyes, and explosives, among others.1-3. The NO yield can reach 90−98 % depending on the operation parameters, rendering it one of the most efficient catalytic reactions.1 However, Pt loss through volatilization (0.05-0.3 g per ton of nitric acid produced) at high-temperature and strongly oxidizing atmosphere results in significant catalytic performance decays and high operating costs.4 Moreover, nitric acid production is the largest source of N2O emission in the chemical industry, equivalent to 125 million tons of CO2 per year in terms of greenhouse effects.5 The above economic challenges and environmental concern stimulate research towards developing cost-efficient catalysts and new processes with favourable NO yield while reducing N2O emission.
Herein, we proposed and validated the first chemical looping ammonia oxidation (CLAO) process (Figure 1). Selectively catalytic ammonia oxidation to nitric oxide via a novel chemical looping system was proposed and demonstrated in absence of noble metal catalyst. Among the many metal oxide redox catalysts investigated, V2O5 exhibited superior NH3 conversion at a substantially lower temperature with an outstanding NO selectivity (99.8%), outperforming the expensive commercially applied Pt−Rh gauzes. In addition, the selectivity to undesirable N2O was largely suppressed. Repeatable NH3 conversion and product selectivity were achieved during consecutive redox cycles over 1300 min. The underlying mechanism was investigated by both operando and ex situ characterization techniques complemented with DFT calculations. Results show that the proposed CLAO process operates via a modified, temporally separated MvK redox mechanism featuring a reversible V5+/V4+ redox cycle. The vanadyl (V=O) centers act as active sites for NH3 oxidation, leading to the formation of the oxidation products. While both V=O and doubly coordinated oxygen (OII) participate in the hydrogen transfer process. The superior activity and selectivity can be attributed to the low activation energies for the successive hydrogen abstraction, facile NO formation and V=O lattice oxygen extraction.
This research effort was led by Prof. Xiaodong Wang from the group of http://www.taozhang.dicp.ac.cn/ at Dalian Institute of Chemical Physics, Chinese Academy of Sciences (Dalian, China) in collaboration with Prof. Fanxing Li of https://www.cbe.ncsu.edu/ligroup/ at North Carolina State University (NC, USA).
More details on this work can be found here: “Selective catalytic oxidation of ammonia to nitric oxide via chemical looping” in Nature communications (https://rdcu.be/cGvD3).
References
- A. I. V.A. Sadykov, I.A. Zolotarskii, L.N. Bobrova, A.S. Noskov, V.N. Parmon, E.A. Brushtein,T.V. Telyatnikova, V.I. Chernyshev, V.V. LuninC., Falter, Appl. Catal. A, 2000, 204, 59-87.
- Schäffer, V. A. Kondratenko, N. Steinfeldt, M. Sebek and E. V. Kondratenko, J. Catal., 2013, 301, 210-216.
- Perez-Ramirez and B. Vigeland, Angew. Chem., Int. Ed., 2005, 44, 1112-1115.
- Hannevold, O. Nilsen, A. Kjekshus and H. Fjellvåg, Appl. Catal. A, 2005, 284, 163-176.
- Pérez-Ramı́rez, F. Kapteijn, K. Schöffel and J. A. Moulijn, Appl. Catal. B, 2003, 44, 117-151.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in