Challenges and Innovations in Detecting Critical Nuclei in Phase Transitions

The detection of critical nuclei in phase transitions is a subject of profound significance in the realm of physics, materials science, and other applied sciences. The formation of a critical nucleus is theoretically expected as the seminal event that dictates the kinetics and pathway of a phase transition, such as the ice formation and crystallization of proteins. By detecting and studying these critical nuclei, it can reveal the microscopic mechanisms governing the behavior of materials under different conditions. This understanding is not just academic, it also has profound implications for our comprehension of many natural phenomena and the laws of thermodynamics. For instance, the formation of ice nuclei in clouds is a critical factor in the precipitation process.
Despite its importance, it is exceedingly difficult to directly detect the forming of critical nuclei in experiments. The difficulties primarily arise from the inherent nature of forming critical nuclei in both the small length scale (a few nanometers) and the fast time scale (nanoseconds). The rare and random occurrence of forming critical nuclei also bring additional difficulties in experiments. Currently, molecular dynamics (MD) simulations have provided key insights into forming critical nuclei for enhancing our understanding of nucleation phenomena. However, the lack of experimentally detecting the critical nuclei in phase transitions has been a major obstacle, hindering our understanding and ability to manipulate these processes effectively.
To address the challenges in detecting critical nuclei in phase transitions, our research team has developed a novel method using graphene oxide nanosheets with the narrowly-distributed sizes to probe the critical nucleus size in ice formation (Nature 2019, 576, 437-441). This method showed that the capability of these nanosheets in facilitating ice nucleation had an obvious transition at a specific value of the product of the lateral size of the nanosheets with the supercooling of occurring ice nucleation. Our experimental observations, coupled with theoretical calculations, suggest that the lateral size of the nanosheets mirrors the dimension of the critical ice nucleus which is inversely proportional to the supercooling temperature. Specifically, for larger nanosheets, the critical ice nucleus forms on their surface, leading to ice formation behaviors that align with the predictions of classical nucleation theory. In contrast, for smaller nanosheets, the ice nucleus changes its shape while meeting with the edges of nanosheets in forming a larger-volume critical ice nucleus with a significantly higher free-energy barrier for nucleation and thus diminishing the facilitating effect of the graphene oxide. Our findings not only provide crucial experimental evidence of the existence and temperature-dependent size of the critical ice nucleus but also promise a general method to probe the critical nuclei in a wide range of other nucleation processes. For example, the critical nucleus size of the metal-insulator phase transition was directly measured by introducing size-controlled nanoscale nucleation seeds. This advancement opens new avenues for understanding and manipulating nucleation in various systems, potentially leading to significant scientific and technological breakthroughs.
Clathrate hydrates, where guest molecules are trapped in water nanocages, are increasingly used in various fields. Controlling their formation is key for advancing hydrate-based technologies, yet the microscopic mechanisms of their nucleation are still not well understood. Few knowledge, especially in experiments, about the critical nuclei in clathrate hydrate formation is known. Based on the developed method used in observing critical nuclei in ice nucleation, our current work focuses on probing the critical nucleus size of tetrahydrofuran (THF) clathrate hydrate from its aqueous solution by employing the anchored nanoparticles on substrate surfaces (Fig.1). By assessing THF the clathrate nucleation-controlling ability of uniform-sized nanoparticles, we have made significant strides.
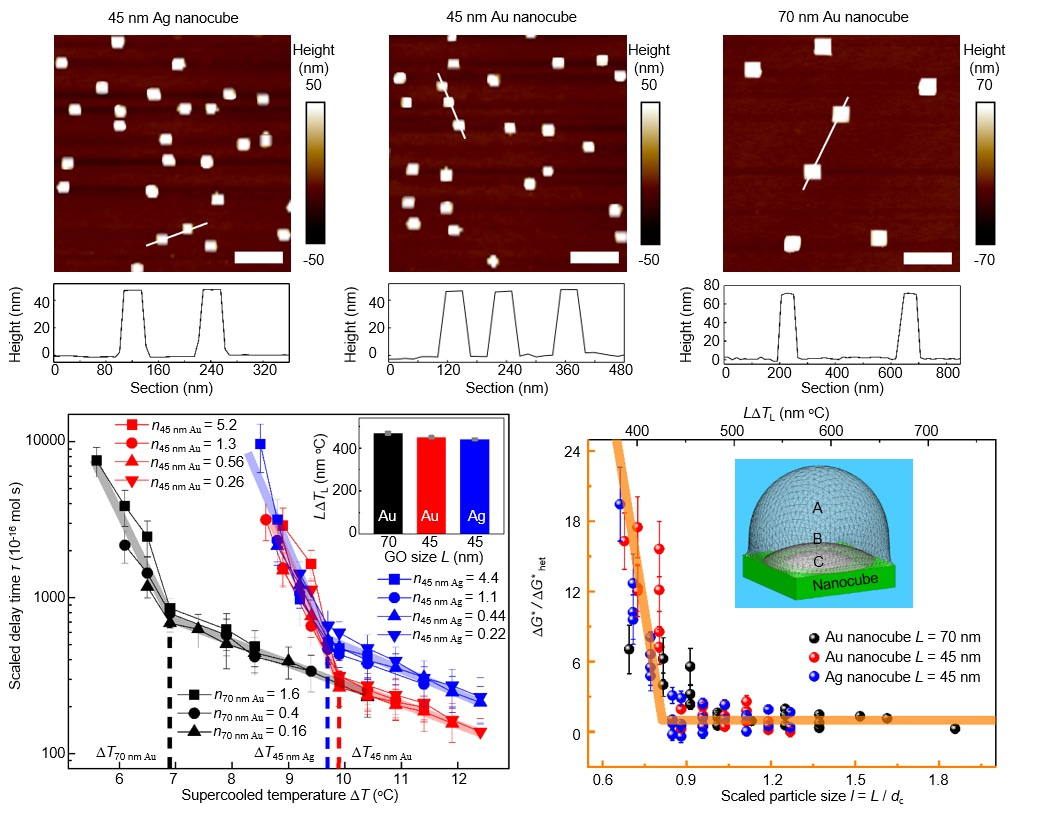
Fig. 1 Abrupt transitions of the THF clathrate nucleation activity of Ag/Au nanocubes.
The key advances in this work are reported as the following:
· We employed uniformly-sized nanoparticles as nanoprobes to experimentally measure the critical nucleus size in THF clathrate nucleation. These nanoparticles exhibited an abrupt change in nucleation capability at a specific supercooling determined by the size of the nanoparticle. Significantly, we observed that the free energy barrier undergoes a substantial alteration when the nanoparticles are approximately the same size as the critical nucleus.
· This finding implies that the nucleation of THF clathrate hydrates involves the formation of a critical nucleus, the size of which is inversely proportional to the degree of supercooling. We established a relationship between the spherical diameter of the critical nucleus and supercooling, approximately dcritical × ΔT ≈ 600 nm oC. This dimension is notably larger than what has been observed in ice nucleation, which indicates a more complicated microscopic mechanism in forming the critical nucleus from the surrounding supercooled solution than that in forming ice nucleus from supercooled water. This contributes significantly to the microscopic understanding of clathrate hydrate nucleation.
· Moreover, the method we have developed, which utilizes nanoparticles as a probing tool, has been proven to be broadly applicable and effective in studying the critical nuclei involved in numerous first-order phase transition processes. This technique extends the scope of phase transition research and provides a new lens through which to examine these critical and often elusive nucleation phenomena.
Looking ahead, our research journey is far from complete. A critical area for future exploration involves the verification of certain pre-nucleation steps. These preliminary stages are hypothesized to influence the structure and characteristics of the solution surrounding the critical nucleus, potentially diverging from those observed in the macroscopic solution. Furthermore, the innovative approach we have developed for probing the size of critical nuclei in nucleation processes holds immense promise for application across a broad spectrum of first-order phase transition systems. This strategy could be adapted to study various materials and conditions, ranging from metallurgical to biological systems. By doing so, we can extend our understanding beyond the confines of clathrate hydrates and ice nucleation, potentially uncovering universal principles that govern phase transitions in diverse environments.
More details can be found in our paper "Probing the critical nucleus size in tetrahydrofuran clathrate hydrate formation using surface-anchored nanoparticles" published in Nature Communications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in