Changes at glutamate tripartite synapses in the prefrontal cortex of a new animal model of resilience/vulnerability to acute stress
Published in Neuroscience

Stress is considered a primary risk factor for neuropsychiatric disorders [1, 2]. Accordingly, many animal models of psychopathology are largely based on exposure to validated chronic stress protocols. However, the investigation of the long-term consequences of acute stress has found structural and functional changes that often resemble those induced by chronic stress. Using a standard protocol of acute inescapable footshock (FS) stress, we have previously shown that traumatic stress can induce both rapid and long-lasting structural and functional alterations in rat prefrontal cortex (PFC) [3–5].
In the present work, we introduce a new rodent stress model based on acute inescapable FS stress, where male rats were deemed resilient/vulnerable based on their behavior in the sucrose intake test for anhedonia, performed 24 h after acute stress. This approach allowed us to identify early functional, molecular, and morphological determinants of stress resilience/vulnerability at tripartite glutamate synapses in the prefrontal cortex (PFC). Our study has recently been published in Translational Psychiatry with the title: “Changes at glutamate tripartite synapses in the prefrontal cortex of a new animal model of resilience/vulnerability to acute stress”.
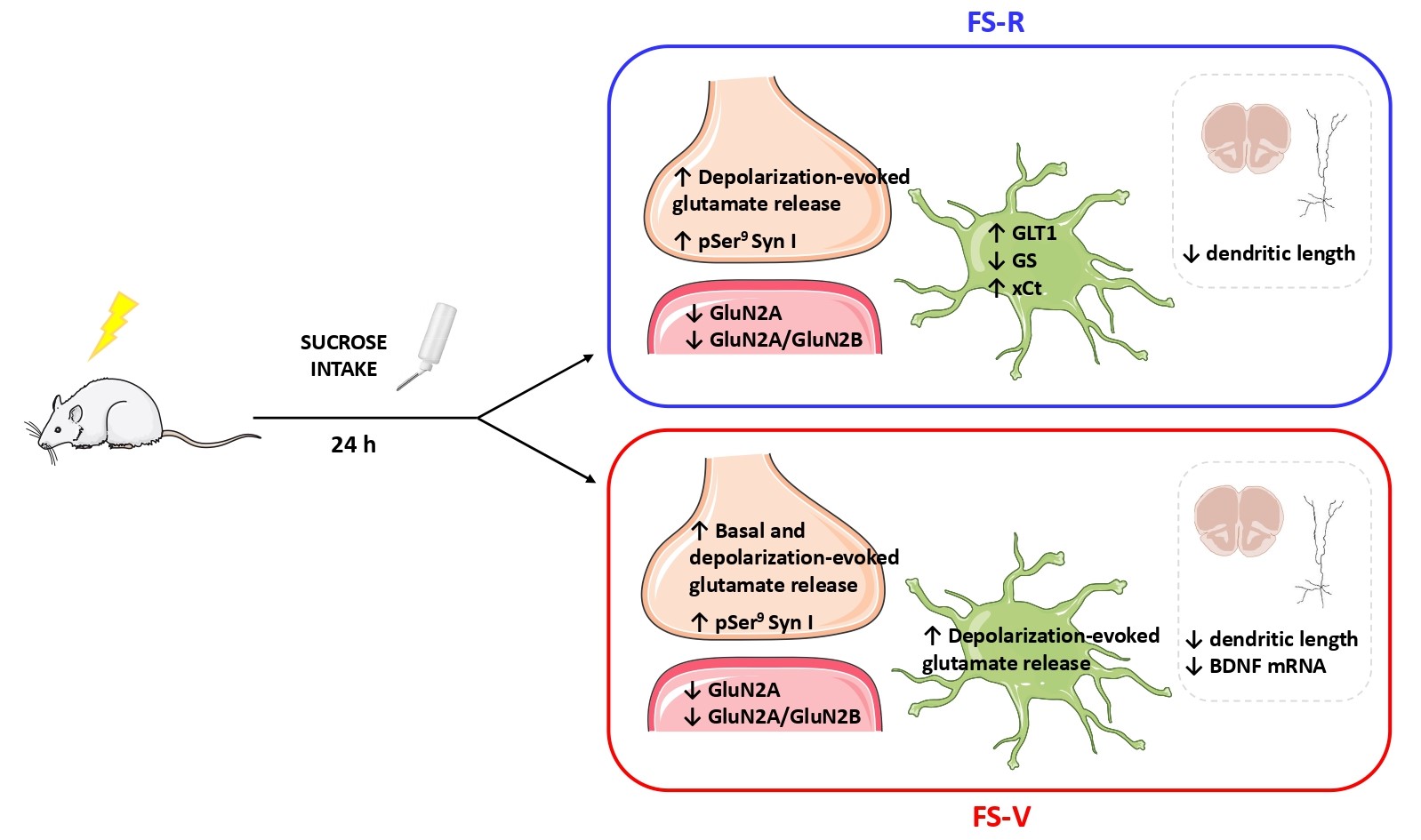
As for functional characterization, we focused on tripartite glutamatergic synapses and dissected neuronal and glial determinants in response to acute stress by studying glutamate release from synaptosomes and gliosomes. Synaptosomes derive from synaptic boutons and varicosities of the axonal processes, maintain the complexity of the presynaptic nerve terminals, and represent the main site of neurotransmitter release. Gliosomes are a subcellular preparation originating from the perisynaptic astrocytes regions. They express proteins involved in the release machinery, and contain vesicles competent for exocytosis, neurotransmitter receptors, and transporters.
We found that depolarization-evoked glutamate release and synapsin I phosphorylation at Ser9 (known to facilitate presynaptic release) were increased in both resilient and vulnerable animals, while basal neuronal glutamate release was increased in the PFC of vulnerable rats only. Basal release of glutamate plays a central role in the maturation and stability of synaptic networks, controlling spike timing, maintaining synaptic strength, regulating postsynaptic responsiveness, and seemingly inhibiting dendritic protein translation. Therefore, its increase could be suggestive of further relevant synaptic changes related to a vulnerability trajectory in the stress response.
At the same time, acute FS decreased the expression levels of the GluN2A subunit of NMDA receptors in PFC synaptic membranes and reduced apical dendritic length and complexity of prelimbic PFC pyramidal neurons in both resilient and vulnerable animals. Nevertheless, BDNF mRNA expression was selectively reduced only in vulnerable rats. On the other hand, depolarization-evoked glutamate release from astroglia perisynaptic processes (gliosomes) was selectively increased in the PFC of vulnerable rats. We then measured the expression of astrocytic proteins related to glutamate homeostasis and found that GLT1 and xCt levels increased while GS expression diminished in purified gliosomes from the PFC of resilient rats.
In the present study we report the first results obtained with a new rodent model of acute stress, envisaged to identify early determinants of resilient vs. vulnerable trajectories of the stress response. Future studies are needed to draw a comprehensive time-dependent behavioral characterization of resilient and vulnerable animals in the days and weeks following stress exposure, thus analyzing specific behavioral phenotypes which could clarify the translational relevance of the model. Sex differences should also be addressed.
Importantly, the purification of synaptosomes and gliosomes allowed for studying functional and molecular changes of the synaptic and perisynaptic moieties of the tripartite glutamatergic synapse in the PFC of vulnerable and resilient animals. Indeed, our results showed that PFC synaptosomes and gliosomes from rats vulnerable and resilient to acute FS displayed a different response to stress, suggesting that neurons undergo early modifications after FS, with astroglia entering the play later, thus suggesting that the observed astroglia modification may be primed by neuronal modification. Alternatively, the molecular changes occurring in PFC gliosomes might be responsible for adaptation, thus assigning astrocytes a central role in avoiding a maladaptive response progression in resilient rats. To detect why this astrocytic attempt failed in vulnerable rats could reveal new mechanisms involved in PTSD and deserves further investigation.
Overall, we could identify early determinants of a maladaptive response leading to behavioral vulnerability to stress. The study of these mechanisms promises to help in the identification of key mediators of pro-adaptive versus maladaptive trajectories.
References
- Sanacora G, Yan Z, Popoli M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci. 2022;23:86–103.
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363.
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute Stress Increases Depolarization-Evoked Glutamate Release in the Rat Prefrontal/Frontal Cortex: The Dampening Action of Antidepressants. PLoS One. 2010;5:e8566.
- Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry. 2014;19:433–443.
- Musazzi L, Tornese P, Sala N, Popoli M. Acute stress is not acute: sustained enhancement of glutamate release after acute stress involves readily releasable pool size and synapsin I activation. Mol Psychiatry. 2017;22:1226–1227.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in