Characterization of the mouse cardiac conduction system
Published in Protocols & Methods
Cardiovascular disease is the single leading cause of death in developed countries. There are a plethora of pathological conditions leading to cardiac arrest and sudden cardiac death. Among them are primary electrical diseases of the heart such as channelopathies, but also structural cardiac diseases. A prominent example is sinus node dysfunction, a pathophysiological condition with drastically limited treatment options that is the main cause of pacemaker implantation. Therefore, diagnostic procedures to assess the function of the cardiac conduction system are of great clinical importance. Furthermore, many cardiac diseases have in common that they ultimately lead to cardiac arrhythmia, most strikingly to torsade de pointes arrhythmia and ventricular tachycardia. Cardiac arrhythmias, in particular ventricular arrhythmia, are potentially fatal. Consequently, there is clinical need to understand cardiac diseases leading to arrhythmia. To elucidate the underlying mechanisms of these cardiac arrhythmias, preclinical animal models - especially genetic mouse models - are immensely important.
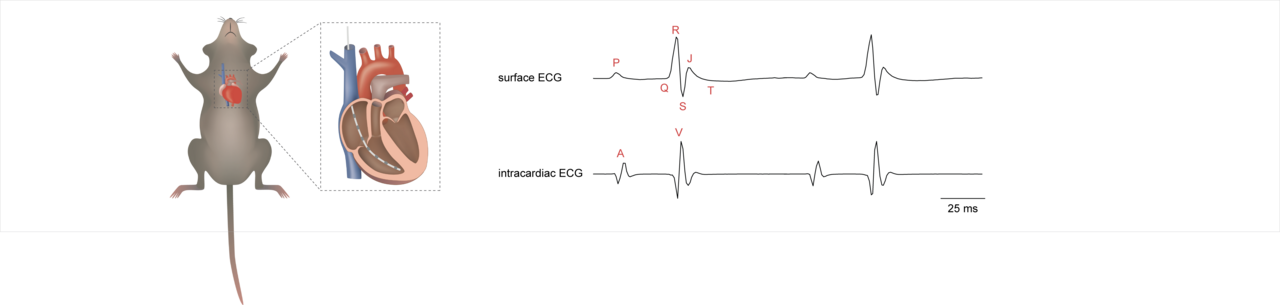
In human cardiology, right heart catheter-based electrophysiological procedures are performed in order to diagnose and distinguish between different types of cardiac arrhythmia. Based on the idea of performing a similar procedure in genetically modified mice, we screened the literature for appropriate protocols. The outcome was a whole series of human protocols and only a few very specialized protocols for mice.
Starting from this initial background we combined, adapted, optimized, and validated different protocols from the literature to be able to use them for in vivo and ex vivo application in the mouse heart. After successful implementation of the method in two independent research projects, we finally decided to compose a comprehensive protocol for electrophysiological investigation of the mouse heart in order to characterize the cardiac conduction system, and to determine susceptibility to atrial and ventricular arrhythmias. Our goal was to create a single manuscript that would both serve as a detailed experimental guide for beginners and as a theoretical and practical reference for experienced scientists.
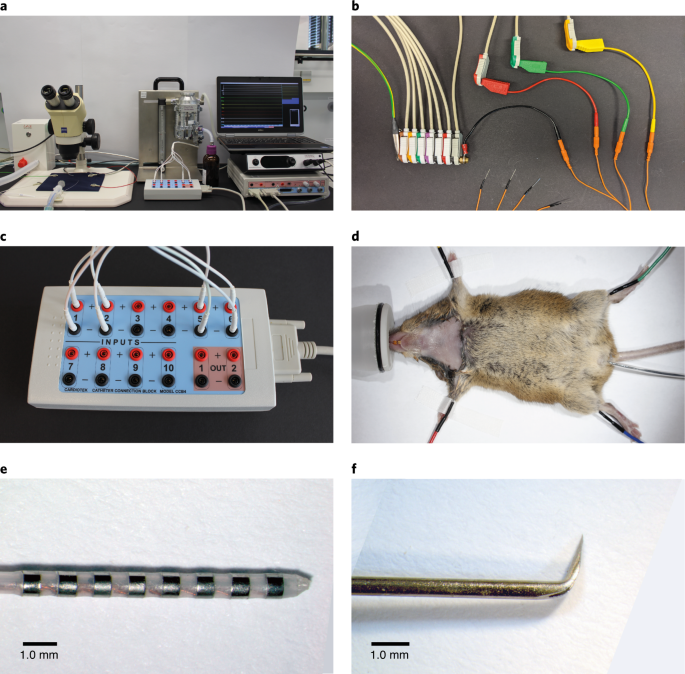
Our protocol provides complete guidance through the whole procedure and offers step-by-step instructions for setting up the acquisition system, performing catheter placement surgery, and applying a comprehensive set of programmed electrical stimulation protocols. These stimulation protocols enable determination of parameters such as sinoatrial conduction time (SACT), sinus node recovery time (SNRT), AV-nodal conduction properties, Wenckebach periodicity, refractory periods, and arrhythmia vulnerability. Furthermore, we include details of ex vivo EPS, which provides important insights into intrinsic cardiac electrophysiology without external influences from humoral and neural factors. In addition, we describe a heart preparation with intact innervation by the vagus nerve that can be used as an ex vivo model for vagal control of the cardiac conduction system. Finally, we precisely describe data analysis and provide broad theoretical background information, which we believe is very helpful for interpreting the results obtained.
We believe that our protocol is of general interest for cardiac electrophysiologists working in basic, translational, or drug development research. The technique yields highly reliable results and can be used for phenotyping of cardiac disease models, elucidating disease mechanisms, confirming functional improvements in gene therapy approaches, or for drug and toxicity testing. The protocol is specifically tailored to analyze preclinical mouse models and to investigate mechanisms underlying disorders in cardiac impulse formation and propagation, which can lead to potentially fatal cardiac arrhythmia.
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in