Chemotherapy induces cell plasticity; controlling plasticity increases therapeutic response
Published in Cancer
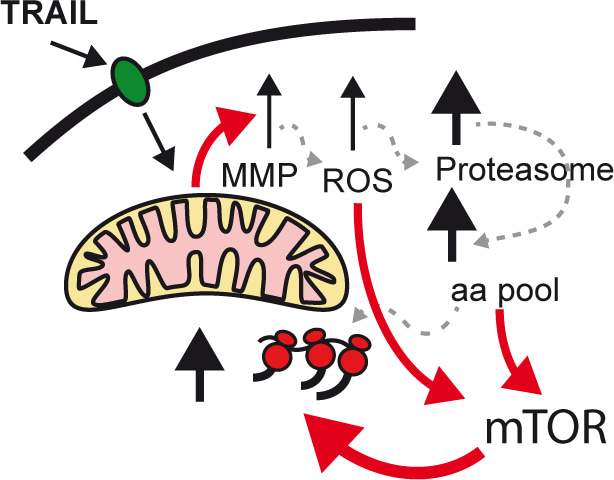
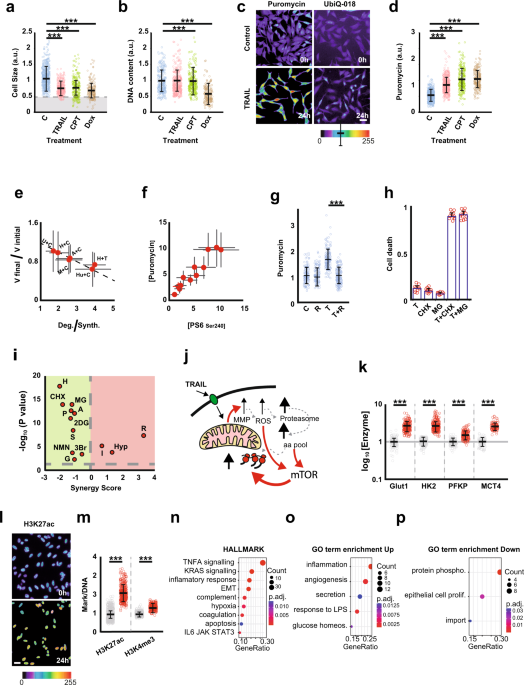
In our lab, we study fractional killing chemotherapy. In this study, we found that the cells resisting the therapy were smaller than the original cells, with even tiny cells appearing. Investigating the nature of this reduction in size, we found that it is due to an altered balance between protein degradation and protein synthesis, in favour of degradation. Thus, chemotherapy triggers proteolysis to a greater extent than protein synthesis. Probing the molecular mechanisms responsible for this behaviour, we found that BAX (a protein involved in the early stages of apoptosis), by interacting with Complex I of the electron transport chain, inhibits cellular respiration, resulting in the production of Reactive Oxygen Species (ROS). The increase in ROS activates proteolysis, which causes the amino acid pool to increase within the cell. And amino acids, in addition to being precursors of protein synthesis, activate mTOR resulting in a generalised increase in protein synthesis.
Activation of protein turnover is essential for chemoresistance, because when we inhibit either phase (synthesis/degradation), we synergistically increase cell death. This result is very interesting because it opens up the possibility of using combinations of chemotherapy with inhibitors of protein synthesis or degradation.
But what are the consequences for the cell to be smaller and have a higher protein turnover? In our opinion, this transformation is essential to increase the cellular plasticity of resistant cells. For two reasons. First, by having a higher protein turnover than untreated cells, they can change their phenotype more rapidly. Second, because they are smaller, they need to invest fewer resources to change the concentration of any given protein than a large cell. In short, this transformation greatly facilitates cell plasticity.
In addition to the reduction in size triggered by the apoptotic cascade, the cell suffers a respiratory deficit which it makes up for by activating glycolysis. The latter process is a source of precursors for the enzymes responsible for epigenetic modification of histones, Acetyl coA and S-Adenosyl Methionione. Thus, chromatin modifications are increased, facilitating transcriptional reprogramming, as we have shown to occur in the treated cells.
Transcriptomic analysis was interesting, as it showed the activation of genes involved in processes such as stress, inflammation, immune evasion, interaction with neutrophils, epithelial-mesenchymal transition, metastasis, etc.
The picture that emerges is that when chemotherapy does not kill, it makes cells more plastic and has a greater metastatic potential. Thus, when we interfere with protein turnover, which is essential for cellular plasticity, we synergistically increase the efficacy of treatments.
Follow the Topic
-
Signal Transduction and Targeted Therapy

This is an international, peer-reviewed, open-access journal publishing articles related to signal transduction in physiological and pathological processes, alongside signal transduction-targeted therapeutics in the form of biological agents and small molecular drugs used to treat human diseases.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in